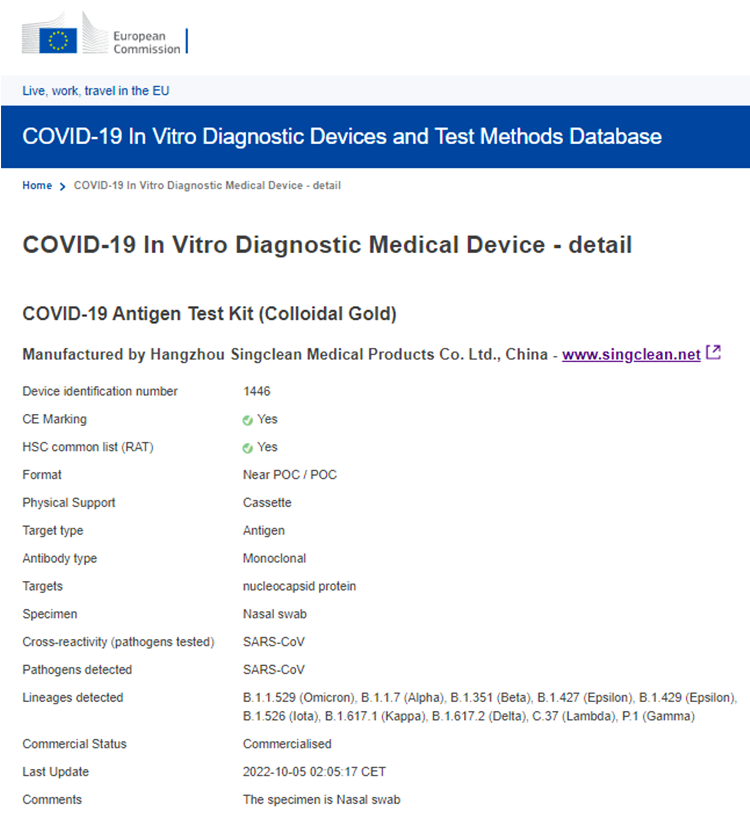
On Oct 5th 2022, Singclean COVID-19 Antigen Test Kit (Colloidal Gold) Nasal Swab is included in the EU Common List!

With the continuous development of Covid-19 and the raging of new mutant strains in the world, the demand for Covid-19 detection reagents in the international market is strong, and the Covid-19 Antigen Test Kit has become the "new favorite" for export.
In this context, the HSC (Health Security Committee) established a technical working group on COVID-19 diagnostic tests in May 2021 to review the proposal for EU national COVID-19 manufacturers and those that need to be included in the EU Common List of Rapid Antigen Tests.
The document requires that the conditions for inclusion in the general list of COVID-19 rapid antigen tests are:
1. Carry CE Mark;
2. Meet the minimum performance requirements: sensitivity>90%, specificity>97%;
3. Validated for use with COVID-19 in at least one Member State, providing details of the methods and results of these validation studies, such as the type of samples used for validation, the environment in which the tests were evaluated, and where required sensitivity criteria or other performance indicators aspects can be satisfied.
At the same time, for the rapid antigen test results to be mutually recognized among member states, the Health Safety Committee (HSC) clarified that at least 3 member states should have verified and used a rapid antigen test in the above common list in practice, then the Only the test results can be mutually recognized. The so-called EU HSC Mutual Recognition List (Mutual Recognition List) comes from this.
Because of our strict quality control system and experienced registration team, we are honored to be trusted and included in Eu Common list!.
Singclean COVID-19 Antigen Test Kit (Colloidal Gold) Nasal Swab
EU Common List - https://covid-19-diagnostics.jrc.ec.europa.eu/devices/detail/1446
Intended Use
COVID-19 Test Kit (Colloidal Gold Method) is a solid phase immunochromatographic assay for the rapid, qualitative detection of antigens to the 2019 Novel Coronavirus in the human nasal cavity
PERFORMANCE CHARACTERISTICS:
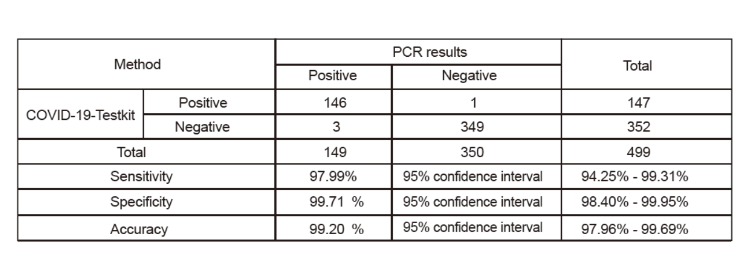
1. Clinical Sensitivity, Specificity, and Accuracy
The results of the COVID-19 Test Kit(Colloidal Gold Method) were compared to the results of RT-PCR assays for SARS-CoV-2 in nasal swab specimens. A total of 499 nasal cavity specimens were tested in this study. The COVID-19 clinical specimens contain specimens from individuals with symptoms within 7 days. The results of the test reagent and control reagent both were 350 negative specimens and 149 positive specimens. The sensitivity and specificity calculated were valid in this study.
-
singclean | 2022-10-11
 Singclean Medical to Showcase Advanced Aesthetic Solutions at Dubai Derma 2026Singclean Medical is set to participate in Dubai Derma 2026, one of the most prominent dermatology and aesthetic medicine exhibitions in the Middle East, taking place from March 31 to April 2, 2026, at the Dubai World Trade Centre (DWTC), UAE. The event serves as a key regional hub, bringing together dermatologists, aesthetic practitioners, and industry leaders from across the globe.
Singclean Medical to Showcase Advanced Aesthetic Solutions at Dubai Derma 2026Singclean Medical is set to participate in Dubai Derma 2026, one of the most prominent dermatology and aesthetic medicine exhibitions in the Middle East, taking place from March 31 to April 2, 2026, at the Dubai World Trade Centre (DWTC), UAE. The event serves as a key regional hub, bringing together dermatologists, aesthetic practitioners, and industry leaders from across the globe. -
singclean | 2022-10-11
 Singclean Medical to Showcase Regenerative Aesthetic Innovations at AMWC 2026Singclean Medical will participate in the Aesthetic & Anti-Aging Medicine World Congress (AMWC) 2026, taking place from March 26 to 28, 2026, at the Grimaldi Forum in Monaco. As one of the most influential global events in aesthetic medicine, AMWC gathers leading physicians, researchers, and industry pioneers to exchange insights on cutting-edge technologies and emerging trends.
Singclean Medical to Showcase Regenerative Aesthetic Innovations at AMWC 2026Singclean Medical will participate in the Aesthetic & Anti-Aging Medicine World Congress (AMWC) 2026, taking place from March 26 to 28, 2026, at the Grimaldi Forum in Monaco. As one of the most influential global events in aesthetic medicine, AMWC gathers leading physicians, researchers, and industry pioneers to exchange insights on cutting-edge technologies and emerging trends. -
singclean | 2022-10-11
 2026 Singderm Global Academic Exchange Program First Stop Launches—— China-Thailand Aesthetic Injection Exchange& SymposiumFrom January 22nd to 23rd, Singderm successfully hosted the China-Thailand Aesthetic Injection Exchange& Symposium event, marking a significant step forward in promoting international collaboration within aesthetics. As a key strategic partner of Singderm in the Thai market, Pola Group invited a distinguished delegation of local experts, including: Dr. Rosalyn, Professor, Department of Anatomy, Dhurakij Pundit University, Bangkok Dr. Grace, Technical Director, Doctor Grace Clinic Dr. Jonny, Technical Director, Doctor Jonny Clinic The program was designed to deepen China-Thailand professional dialogue on injectable aesthetics and foster the integration and evolution of Eastern aesthetic concepts and techniques.
2026 Singderm Global Academic Exchange Program First Stop Launches—— China-Thailand Aesthetic Injection Exchange& SymposiumFrom January 22nd to 23rd, Singderm successfully hosted the China-Thailand Aesthetic Injection Exchange& Symposium event, marking a significant step forward in promoting international collaboration within aesthetics. As a key strategic partner of Singderm in the Thai market, Pola Group invited a distinguished delegation of local experts, including: Dr. Rosalyn, Professor, Department of Anatomy, Dhurakij Pundit University, Bangkok Dr. Grace, Technical Director, Doctor Grace Clinic Dr. Jonny, Technical Director, Doctor Jonny Clinic The program was designed to deepen China-Thailand professional dialogue on injectable aesthetics and foster the integration and evolution of Eastern aesthetic concepts and techniques.




