Singderm® Calcium Hydroxyapatite Hybrid Filler
Caution: General law restricts this device to sale by or on the order of a licensed healthcare professional or properly licensed practitioners
BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY
1. DESCRIPTION
Singderm®️ Dual is sterile. biodegradable. nonpyrogenic, viscous, opaque, semisolid, latex-free for dermal fillers, it is formulated in a physiologic buffer, consists of synthetic calcium hydroxyapatite microspheres at a concentration of 56%, cross-inked hyaluronic acid (HA) at a concentration of 20 mg/mL, and 0.3% Lidocaine Hydrochloride, Sodium hyaluronate is derived from bacteria fermentation. Singderm® Dual is presented in a graduated, pre-fed, disposable syringe. Each box contains one syringe and an instruction leaflet, The contents of the Singderm® Dual syringe are sterilized by moist heat. And the needles are sterilized by ethylene oxide.
2. INDICATIONS
Sinaderm Dual is intended for deep dermal to subdermal implantation for the correction of moderate to severe facial wrinkles, folds, and/or volume increase, such as nasolabial folds, marionette lines, jawline, cheeks, and nose.
3. MODE OF ACTION
The injection of the Sinaderm Dual can produce an immediate corrective effect, When the gel carer is absorbed, calcium hydroxyapatite particles act as a scaffold for new tissue formation and collagen deposition to adsorb fibroblasts, stimulate fibroblasts to secrete collagen (mainly type l and ll) and various elastic fibers through direct mechanical conduction, promote the growth of collagen fibers, and restore skin elasticity and support. Over time, calcium hydroxyapatite particles are broken down into calcium and phosphate ions, which are slow-eliminated through the body's normal metabolic pathways.
4. CONTRAINDICATIONS
Singderm® Dual is contraindicated for patients with severe allergies manifested by a history of anaphylaxis or history or presence of multiple severe allergies.
Singderm® Dual contains trace amounts of gram-positive bacterial proteins and is contraindicated for patients with a history of allergies to such material.
Singderm® Dual is not to be used in patients with known hypersensitivity to any of the components.
Singderm® Dual is not intended to be used in patients with known hypersensitivity to lidocaine or anesthetics of the amide type.
Singderm® Dual is contraindicated for patients with bleeding disorders.
Singderm® Dual is contraindicated for the area of the periorbital, glabella, lips, and perioral region.
5. WARNINGS
The product must not be injected into blood vessels, the introduction of Sinaderm® Dual into the vasculature may lead to occluding the vessels ischemia, infarction, or embolization.
Use of Singderm® Dual in any person with active skin inflammation, infection, or tumor in or near the treatment area should be deferred until the underlying process has been controlled.
Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because hyaluronan can precipitate in their presence.
6. PRECAUTIONS
Singderm® Dual is packaged for single-patient use. Do not resterilize. Do not reuse. Do not use it if the package is opened or damaged. Do not use if the syringe end cap or syringe plunger is not in place.
As with all transcutaneous procedures, dermal filler implantation carries a risk of infection. Standard precautions associated with injectable materials should be followed.
Singderm® Dual is to be used as supplied. Modification or use of the product outside the instructions for Use may adversely impact the sterility, homogeneity, and performance of the product and it can therefore no longer be assured.
The recommended injection volume is with a maximum of 6mL per year.
Singderm® Dual should not be mixed with other products. Treatment with the product in combination with drugs or other medical devices has not been tested.
Due to the presence of lidocaine, the combination of Sinaderm Dual with certain drugs that reduce or inhibit hepatic metabolism (cimetidine beta-blockers.etc.) has not been tested.
The safety and efficacy of Singderm® Dual after dilution have not been evaluated.
Singderm® Dual is intended for use in patients over 18 years of age, the safety for use during pregnancy, in breastfeeding females. or inpatients under 18 years has not been established.
The safety of the same area where already treated with temporary or permanent fiers has not been established.
The safety in patients with known susceptibility to keloid formation, hypertrophic scarring, pigmentation disorders, or the development of
inflammatory skin diseases has not been established.*
Singderm® Dual should be used with caution in patients on immunosuppressive therapy or in diabetes.
Patients who are using substances that can prolong bleeding (such as aspirin, anti-coagulant drugs, anti-inflammatory drugs, and warfarin) may, as with any injection, experience increased bruising or bleeding at injection sites.
Due to the presence of lidocaine, Singderm® Dual should be used with caution in patients showing symptoms of cardiac conduction disorders.
Failure to comply with the needle attachment instructions could result in needle disengagement and/or product leakage at the Luer-lock and needle hub connection.
After use, treatment syringes and needles may be potential biohazards. Handle and dispose of these items in accordance with accepted medical practice and applicable local, state, and federal requirements. Discard needles in sharp collectors.
lf laser treatment, chemical peeling, or any other procedure based on active dermal response is considered after treatment with Singderm® Dual, there is a possible risk of eliciting an inflammatory reaction at the indications site. An inflammatory reaction is also possible if the product is administered before the skin has healed completely after such a procedure.
Serious incidents associated with the injection of Singderm® Dual should be reported to the distributor, manufacturer, and the competent authority.
7. SIDE EFFECTS
The patients must be informed that there are potential side effects associated with implantation of this product, which may occur immediately or may be delayed. These include, but are not limited to:
As with any subcutaneous injection, possible side effects are associated with treatment with dermal fillers, Common adverse events include erythema (redness), edema (swelling), pain, bruising, tenderness, lumps, and itching. Treatment site reactions usually resolve within 24-48 hours, and swelling resolves within a week.
Less common adverse events include: hematoma and seroma. extrusion, induration, nodules, skin pigmentation, fistula formation, inflammatory reaction, infection, allergic reaction, migration, persistent nodules, granulomas, and abscesses.
Another risk is an accidental injection into a blood vessel which can lead to the following complications: vision abnormalities, blindness, stroke, necrosis or permanent scarring.
Patients should promptly inform their healthcare professional if common adverse events do not resolve within the usual period or if they worsen. Some adverse events may require surgical intervention. including drainage of hematomas or seromas and removal of the product in severe cases of allergy, inflammation, hypersensitivity, or infection, Also report if any other adverse event occurs.
8. METHOD OF USE & POSOLOGY
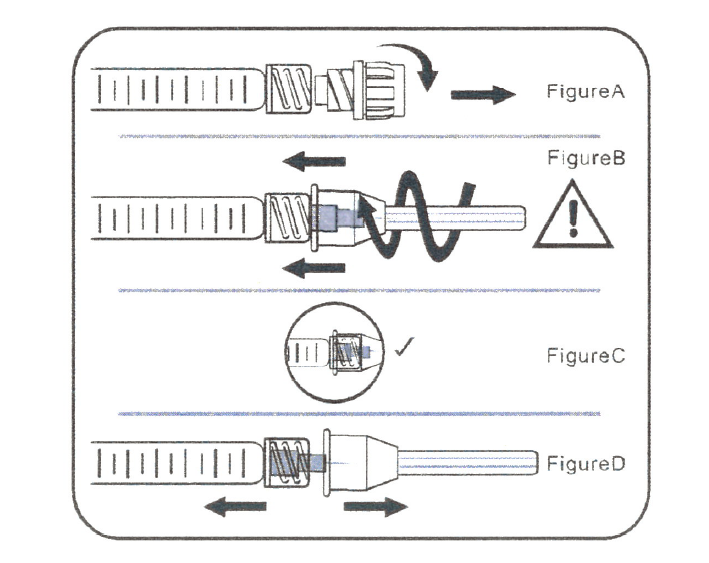
A. Attach Needle to Syringe
STEP 1: Remove tip cap
Hold syringe and pull tip cap off the syringe as shown in Figure A.
STEP 2: Insert needle
Hold the syringe body and firmly insert the hub of the needle into the Luer-lock end of the syringe.
STEP 3: Tighten the needle
Tighten the needle by turning it firmly in a clockwise direction(see Figure B) until it is seated in the proper position as shown in Figure C
NOTE: Continue to tighten until the needle is seated in the proper position.
STEP 4: Remove the needle cap
Hold the syringe body in one hand and the needle cap in the other. Without twisting, pull in opposite directions to remove the needlecap as shown in Figure D.
B. Physician instructions
1.In order to minimize the risks of potential complications, Singderm® Dual should only be used by healthcare practitioners who have appropriate training, and experience, and who are knowledgeable about the anatomy at and around the site of injection, And health care practitioners should fully familiarize themselves with the product. the product educational materials and the entire package insert.
2. Prior to treatment, the patient's complete history should be consulted, and the region to be treated should be carefully evaluated, Patients must be informed about the contraindications, warnings, and possible adverse events of the treatment.
3. Before starting treatment, physician should check the expiration date of the product.
4. Singderm® Dual is a homogeneous gel. Carefully inspect the gel before injecting. Do not use it if you notice particles, discoloration, or signs of fragmentation.
5. The area to be treated should be disinfected thoroughly prior to the injection.
6. Follow the above attaching needle to syringe steps, and depress the plunger rod until the product flows out of the needle.
7. After the first small amount of material has been injected into the patient, wait 3 seconds to allow the lidocaine to take effect before proceeding with the rest of the injection.
8. The injection technique may vary with regard to the angle and orientation of the bevel, the depth of injection, and the quantity administered A linear threading technique, serial puncture injections, or a combination of the two techniques have been used to achieve optimal results. Injecting the product too superficially may result in visible lumps and/or discoloration. Care must be used to avoid intravascular injection regardless of the technique used.
9. The paleness occurs at injection area may indicate that the injection was made into a superficial layer of skin or into a blood vessel. In this case, slop the application and massage the area until the color returns to normal. lf normal color is not restored, the injection process should not be resumed and the use of a vasodilator or other measures should be considered.
10. Inject Singderm® Dual by applying even pressure on the plunger rod while slowly pulling the needle backward. The wrinkle should be lifted and eliminated by the end of the injection. It is important that the injection be stopped just before the needle is pulled out of the skin to prevent material from leaking out or ending up too superficially in the skin.
11. If the needle is blocked, do not increase the pressure on the plunger rod. Instead, stop the injection and replace the needle.
12. The amount injected will depend on the areas which are to be corrected. Do not overcorrect (overfill a contour deficiency because the depression should gradually improve within several weeks as the treatment effect of Singderm® Dual occurs.
13, When injection is completed, the treated site should be gently massaged so that it conforms to the contour of the surrounding tissues.
14. Within the first 24 hours, patients should avoid strenuous exercise, excessive sun or heat exposure, and alcoholic beverages. Exposure to any of the above may cause temporary redness, swelling, and/or itching at the injection sites.
15. The patient should apply an ice pack or cold compresses to the treated area within 24 hours of treatment to reduce redness, swelling, and irritation.
16. After the initial treatment, an additional treatment (from 1 to 2 weeks later) may be necessary to achieve the desired level of correction. If the winkle needs further treatment, the same procedure should be repeated until a satisfactory result is obtained. The need for an additional treatment may vary from patient to patient and is dependent upon a variety of factors such as winkle severely, skin elasticity, and dermal thickness at the treatment site.
9. SPECIFICATIONS
0.5mL,0.8mL.1mL, each syringe ie with a 25G needle and a 26G needle.
10. SHELF LIFE AND STORAGE
Shelf life is 2 years. Store between 2 'C and 25 'C. DO NOT FREEZE