Product name:
Sodium Hyaluronate-Polydeoxyribonucleotide Complex Solution for Injection
Specification:
1.0mL/pc, 1.5mL/ pc, 2.5mL/ pc
Product Performance:
The product is a colorless or white translucent viscous solution, and the water used for its preparation is water for injection.
Structure and composition:
This product consists of prefilled syringe and a complex solution encapsulated in the prefilled syringe. The complex solution consists of non-cross-linked sodium hyaluronate, trace amounts of cross-linked sodium hyaluronate, polydeoxyribonucleotide (PDRN), sodium chloride, disodium hydrogen phosphate, sodium dihydrogen phosphate and water for injection. The product is sterilized by moist heat and is for single use. The product is valid for 2 years.
Indication:
The product is used for injection into dermis. It mainly improves skin elasticity and brightens skin tone through the moisturizing and hydrating effects of materials such as sodium hyaluronate.
Contraindications:
1. It is prohibited to use in patients with autoimmune diseases.
2. It is prohibited to use in patients who are during the outbreak of skin herpes.
3. It is prohibited to use in patients with abnormal coagulation mechanism.
4. It is prohibited to use in patients who are during active skin infection.
5. It is prohibited to use in patients who are during allergic episodes.
6. It is prohibited to use in patients who are allergic to sodium hyaluronate or polydeoxyribonucleotides.
7. It is prohibited to use in pregnant, lactating women and minors.
8. It is prohibited to use in patients with scar-prone constitution.
Installation and Usage Instructions:
This product can only be used in medical institutions officially approved by the local authority, by personnel with relevant professional medical qualifications, use in strictly accordance with the requirements of the product instructions.
1. Before treatment:
The facial skin needs to be surface anesthetized and disinfected. The injection should be performed in operating room and product should not be opened before use. Physicians should evaluate whether this product is suitable for the patient. Patients should be informed of the product's indication, expected treatment outcomes, precautions before and after treatment, contraindications, and possible side effects. Before injection, push the plunger up to remove the air at the front end, and then start the injection.
2. Injection method:
This product should be injected into the dermis of the skin. The injection depth and dosage must be determined based on the patient's actual condition. The correct injection technique of the physician is critical to the treatment effect. Injections can be administered with either a syringe needle or a subcutaneous electronic injector-controlled boost device, which is not supplied in this product.
3. Assembly of injection needle:
This product is used in conjunction with 30G × 1/2” disposable injection needle. First, loosen and remove the syringe cap. Quickly open the package seal of the injection needle. Hold the needle protective cover gently, screw the needle into the syringe with Luer lock, and tighten it. Remove the protective cover of the injection needle, then the treatment can be started. Before injection, check whether the needle and syringe are installed correctly. If abnormal resistance is encountered during injection, stop the injection and reinstall the needle. See the picture below for step-by-step instructions.
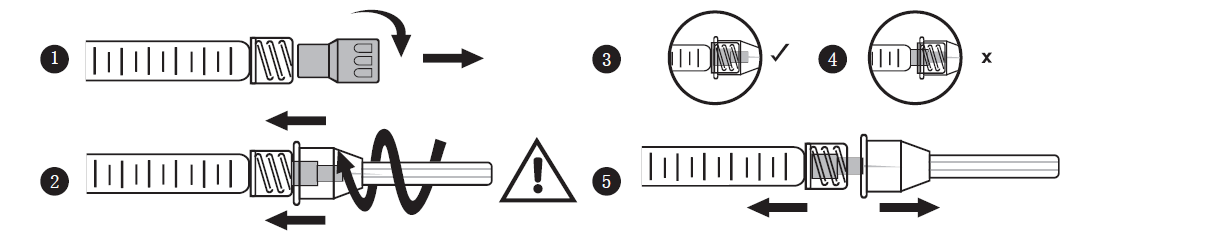
4. Dosage and frequency of use:
The recommended regimen consists of 3 sessions, each time with a 4-week interval. Additional treatments may be given every 4 weeks if the physician deems it necessary based on patient’s skin condition. The recommended dose for a single injection is 2.5 -5mL.
5. Postoperative treatment:
Apply ice for 5 to 10 minutes after injection. Try not to clean or make up within 24 hours to avoid local contamination and infection. Do not drink alcohol or eat irritating food within one week after the injection. For immunocompromised patients, oral antibiotics may be considered as appropriate according to physician's orders. Any abnormalities after use should be reported to the physician.
Expected Adverse Reactions:
1. Pain may occur during the injection process. There are individual differences due to the individual sensitivity threshold, and it also depends on some specific factors, such as the depth of the injection site and physician’s proficiency in injection skills. External application of anesthetics or ice can be considered to reduce pain.
2. If allergic reactions such as rash occur during the injection, injection should be stopped.
3. If skin barrier system is damaged during injection, infection may occur. However, infection can be avoided
by following these measures:
• Before use, confirm that the packaging is intact;
• Strictly disinfect the surgical area;
• Give patients appropriate and reasonable postoperative advice and guidance.
4. After injection, slight edema and redness may occur, which usually disappear in 72 hours.
5. Any serious incident that has occurred in relation to the device should be reported to the manufacturer and the distributors.
Accessories List:
1 piece of syringe (containing sodium hyaluronate polydeoxyribonucleotide complex solution for injection)
1 copy of instruction manual
Warning and Tips:
1. This product is for single use only. Do not sterilize again or use it multiple times.
2. Do not use if the packaging is damaged.
3. Check the product specification, expiration date and lot number before use, and read the instructions carefully.
4. This product cannot be injected into blood vessels.
5. This product cannot be injected into the breast area.
Precautions:
1. This product is packaged in prefilled syringe. Please read the instructions carefully and use with caution. This product does not need to be sterilized again and can be used within the validity period after unsealing as long as the outer packaging is intact. Do not use if the packaging is deformed or damaged.
2. This product is a disposable product. All remaining products and syringes must be discarded in accordance with local law.
3. Each package is individually packaged for one-time use by one person only.
4. Each package is marked with the production date, expiration date, lot number, and specification. If the label falls off or cannot be identified, the corresponding product is prohibited from use.
Storage conditions:
Store at room temperature, do not freeze.
Transport conditions:
Transport at room temperature.
Production Date:
See product label.
Shelf Life:
2 years.
1000.020.02.0003(J)-A0 Issued date: 2024-07-19