Product Name
Sterile Sodium Hyaluronate Solution For GAG Layer Replacement
Indications
For temporary replacement of the GAG layer in the bladder.
Specification
2mg/mL 20mL
Performance, Structure and Composition
This product consists of a prefilled syringe, a catheter connector, and sodium hyaluronate solution encapsulated in the syringe. Among them, sodium hyaluronate solution is composed of sodium hyaluronate, sodium chloride, phosphate buffer saline, and water for injection. The raw material of sodium hyaluronate is obtained through microbial fermentation and does not contain animal or human derived ingredients. The indicated concentration of sodium hyaluronate is 2mg/mL. Sodium hyaluronate solution contained in the prefilled syringe is sterilized with high-temperature steam. Catheter connector contained in the paper plastic bag is sterilized with ethylene oxide.
Description
The glycosaminoglycan(GAG) layer on the luminal surface of the bladder wall is believed to provide a protective barrier against microorganisms, carcinogens, crystals and other agents present in the urine and has been identified as the primary defense mechanism in protecting the transitional epithelium from urinary irritants. Deficiencies in this GAG layer of the bladder epithelium may destroy its barrier function and allow the adherence of bacteria. microcrystals proteins and ions, or the movement of ionic and nonionic solute residues (i.e, urea) across the epithelium, The product has been developed to temporarily replenish the deficient GAG laver on the bladder epithelium, The active substance is highly purified sodium salt of hyaluronic acid (HA).
Principle of Action
The product can supplement patients with sodium hyaluronate and form a protective film. temporarily replacing the deficient GAG layer on the bladder epithelium.
Contraindication
Do not use this product if you have a history of allergy to its ingredients.
Warning
1. Keep out of the reach of children.
2. Do not administer to patients with known hypersensitivity reactions
3. Do not use it if the sterile packaging is damaged.
4. For single use only
5. Discard after use.
Precautions
1. The irrigation into bladder should be provided by a trained medical specialist in specialized premise according to instructions for use.
2. The product has already been sterilized, Please do not sterilize again.
3. Avoid using the product with instruments sterilized with quaternary ammonium salts solutions.
4. As no clinical evidence is available on the use in children, pregnant and lactating women, treatment with the product is not recommended in these patients.
5. Dispose of the product after use in accordance with hospital regulations or environmental requirements
Adverse Event
Adverse reactions related to the product have not been reported yet. Any serious incident that has occurred in relation to the device should be reported to the manufacturer.
Installation instructions
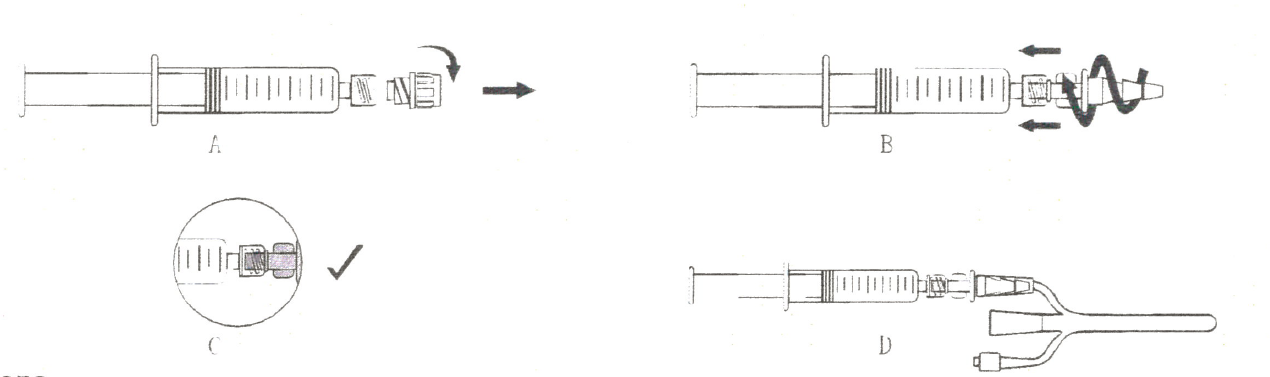
1. Unscrew the protective cap from the top of the syringe (Figure A).
2. Grasp the syringe, accurately insert the catheter connector into the Luer interface end of the syringe (Figure B), and rotate clockwise to tighten the catheter connector until there is no gap (Figure C).
3. Grasp the syringe and catheter flushing chamber with both hands, and insert the catheter connector into the catheter using chamber until there is no gap(Figure D).
Directions
Instill the entire volume of this solution into the bladder after any residual urine has been removed. Discard any unused portion. For best results, the product should be retained in the bladder for as long as possible (a minimum of 30 minutes).
After the product is used., hold the catheter connector and the catheter flushing chamber separately, and rotate the catheter connector clockwise out of the catheter flushing chamber.
Dosage Regimen Recommended
There is evidence that the GAG layer of the bladder is deficient in cystitis. This deficiency contributes to the clinical symptoms in the diseases such as interstitial cystitis, cystitis caused by infections, and trauma. urolithiasis, urinary retention, neoplasia and radiation induced cystitis. To alleviate cystitis associated with these conditions, it is recommended that the product be instilled into the bladder each week for 4 to 6 treatments and then monthly until symptoms resolve. The attending physician, urologist, or radiologist should direct any prophylactic use of the product.
Package
1x20mL Prefilled Syringe; 1xCatheter Connector
Validity Period and Storage Condition
1. Validity period: 3 years.
2. Temperature: 0℃-20℃. Do not freeze.
3. Heavy pressure, direct sunlight, rain and snow immersion should be avoided.
8.57.04.0155-A0 Issued date: 2024-06-25