Microalbuminuria (MAU) Rapid Test Kit (Colloidal Gold)
Instructions For Use
For Self-testing Use
【Product Name】
Microalbuminuria (MAU) Rapid Test Kit (Colloidal Gold)
【Pack Formats and Size】
Strip: 1 pc/box, 25 pcs/cask, 100 pcs /box
Cassette: 1 pc/box, 5 pcs/box, 50 pcs/box
Midstream: 1 pc/box, 20 pcs/box
【Intended Use】
Microalbuminuria (MAU) Rapid Test Kit (Colloidal Gold) is intended for qualitative determination of albuminuria levels in urine in vitro diagnostic. It is used as a supplementary method in the diagnosis of chronic kidney injury(CKI).
【Background】
Microalbuminuria(MAU) is a term to describe a moderate increase in the level of urine albumin. It is also named urine microalbuminuria(UMA or mALB) Albumin is a normal protein in the blood, But under physiological condition sit appear only very small amounts of albumin in the urine. Albumin molecular weight is 70KD, isoelectric point (IEP)is about 4.85.MAU occurs when the kidney leaks small amounts of albumin into the urine, So increased excretion of albumin (microalbuminuria) is an early indicator of glomerular disease.
The researches have showed that MAU can be caused by diabetic nephropathy, hypertension, and cardiac insufficiency.
【Principle】
MAU Rapid Test Kit utilizes colloidal gold labeling and immune chromatography based on the principle of competitive binding to detect of albumin in human urine. During testing, a urine specimen migrates upward by capillary action. Albumin, if present in the urine specimen at an insufficient concentration, will not saturate the binding sites of antibody coated particles in the test strip. The antibody coated particles will then be captured by immobilized albumin conjugate and a visible colored line will show up in the test line region. The colored line will not form in the test line region if the albumin concentration is sufficient because it will saturate all the binding sites of anti- Albumin antibodies. So, a positive urine specimen will not generate a colored line in the test line region because of competition, while a negative urine specimen will generate a line in the test line region.
To serve as an internal procedural control, the control line region (C) is coated with polyclonal Goat Anti-Chicken lgY antibody which will always conjugate binds to Chicken IgY-colloidal gold, so a colored line will always appear in the control line region(C). It is indicated that proper volume of specimen has been added, membrane wicking has occurred and procedural technique is corrected.
【Composition】
Individually packed Strip/ Cassette/ Midstream The main component of the test included MAU antigen, anti-MAU antibody, Goat Anti-Chicken lgY, Chicken lgY, Chloroauric acid, NC membrane.
Dropper(for test cassette)
Introductions for use
Standard Color Card,
【Materials Required but Not Provided】
Timer, Single use specimen collection container
【Storage and Expiry Date】
·Store the Kit at 4~30°℃, keep in a cool and dry place, protected from light. Do not freeze.
·See the package with expiry date. Do not use it after the expiry date.
· It is valid for 30 days for the strips packaged in a cask after opened. And close the cap after used, keep the cap tightly
Use
【Specimen Collection】
·Collect urine specimen according to the conventional laboratory procedures.
·Collect urine specimen by a disposable plastic or glass container which is clean, dry and not contain any preservatives.
·2 hours before collecting the urine specimens, should not consume a large amount of liquid or drinks to prevent getting inaccurate result.
·lf there is sediment at the bottom of the container, please centrifuge, filter, or precipitation and use the supernatant.
·lf there is no time to test, urine specimen can be refrigerated in 2 ~ 8°C for 48 hours. For long term storage, specimens should be kept below -20°C, avoid repeated freezing and thawing of specimens.
·Before testing, refrigerated specimen should be reach to the room temperature, and frozen specimen should be completely.
·All the specimens may be infectious agent or have potentially biological hazard, when collecting or using someone's urine, should pay attention to wear disposable gloves and masks to prevent contacting with others' urine.
【Procedure】
Testing
1.Please read all the information in this IFU before performing the test. Allow the test kit to reach room temperature before use.(20~30°C).
2.Remove the test kit from the foil pouch. The test kit should be used as soon as possible especially at temperature higher than 30°C or highly humid environment.
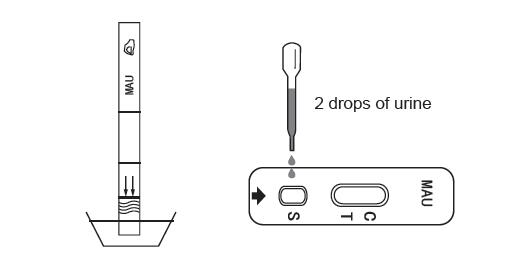
3.strip: Put the test strip in the urine sample at the direction of arrow till the max line at least 10-15 seconds. Do not pass max line. Take the strip out of the specimen and place it on a non-absorbent flat surface.
Cassette: Place the cassette on a clean and level surface. Hold the dropper vertically and transfer 2 full drops of urine (approx.80 μL) to the specimen well (S), and then start the timer. Avoid trapping air bubbles in the specimen well (S).
Midstream: Dip the midstream vertically in the urine specimen for at least 10-15 seconds. Do not pass the plastic part of the midstream when immersing the midstream. Take the midstream out of the specimen and place it on anon-absorbent flat surface.
4. Wait for the colored line(s) to appear. Read the result at 3-5 minutes and then read the resut. Don’t read the result after 10 minutes.
【Result Interpretation】
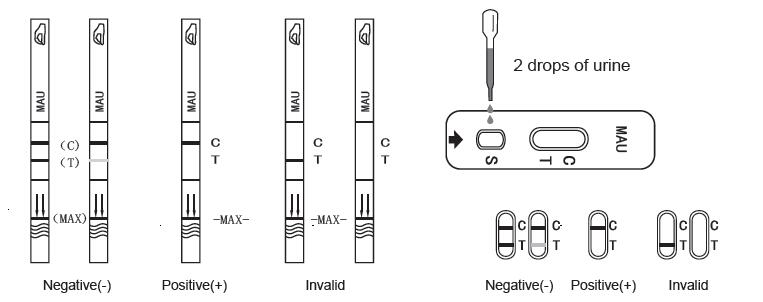
Negative (-)
Two red lines are visible. One is located in the test line region (T), the other is in control line region(C).A negative result with the test indicates the albumin in urine is less then cut off value.
Positive (+)
One colored line appears in the control line region (C).No line appears in the test line region (T).A positive result with the test indicates the albumin in urine is more then cut off value.
It should be considered positive even there is a faint line in T line region, like G3-G4 showed in the Standard Color Card bellow. It indicates the concentration of albumin present in the urine is around the cut off value.
Invalid
There is no red line in control region (C). Incorrect operation or the product has been damaged are the most likely reasons. Please repeat the test with a new test. If the problem still exists, should stop using this batch of product immediately and contact with local suppliers.
【Limitation】
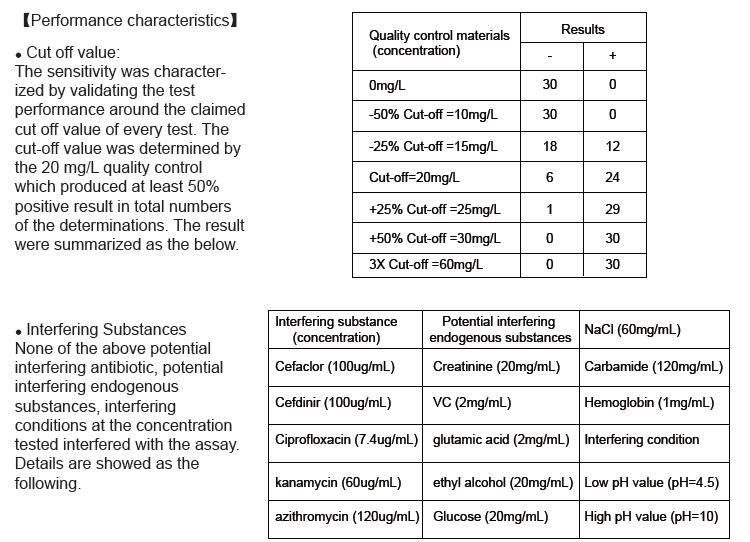
1.The test is only used for qualitative determination of albumin in urine. A positive result with the test indicates the albumin concentration in urine is more than 20mgL, and does not necessarily indicate kidney injury.
2.Only use for in vitro diagnostic, and cannot reused.
3.Please read the result in time, don't read the result after 10 minutes.
4.The test kit provides a presumptive diagnosis. A conformed diagnosis should only be made by a physician after all clinical and laboratory findings have been evaluated.
【Precautions】
1.Only use for in vitro diagnostic, and cannot reused.
2.Do not use if the ponch is damaged, avoiding influence the test results of judgment.
3.Please test in 15 minutes after unpacking to avoid paper get damp and influence the test results.
4.To avoid the cross contamination of samples, don't touch the nitrocellulose(NC) membrane on the product and each test should use new sample container
5.Contaminated urine specimen (e.g. containing bleach or alum) or improper operation cause incorrect results
6.Use the product before expiry date printed in foil pouch.
7.Handle all specimens, used products, packages and droppers as biological activity waste, please do not arbitrarily discard.
Latest version 8.30.04.011 A1 lssued Date: 2020.11.04