COVID-19 Test Kit (Colloidal Gold Method)
For self-testing use Nasal Swab
INTENDED USE
COVID-19 Test Kit (Colloidal Gold Method) is a solid phase immunochromatographic assay for the rapid, qualitative detection of antigen to 2019 Novel Coronavirus in human nasal cavity. This test is used to detect nucleocapsid antigen of the SARS-CoV-2 virus in individuals suspected of having COVID-19. This test is an aid for diagnosis of COVID-19 and only provides a presumptive screening test result for the SARS-CoV-2 virus.
This test is authorized for self-test in individuals:
· Aged 18 years or older
· Aged 2-17 who have their tests supervised by a parent or legal guardian
· Who have covid like symptoms within 7 days.
The test must be used with the nasal swab provided in the kit.
INTRODUCTION
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatically infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
PRINCIPLE
The COVID-19 Test Kit (Colloidal Gold Method) is a colloidal gold immunochromatographic assay. It detects the nucleocapsid protein on the surface of COVID-19.
The test uses COVID-19 (SARS-CoV-2) antibody (test line T) and goat anti-chicken IgY (control line C) immobil- ised on a nitrocellulose strip. The burgundy colored conjugate pad contains colloidal gold conjugated to another COVID-19 (SARS-CoV-2) antibody conjugated with colloid gold and chicken IgY-gold conjugates. When the processed buffer containing the sample is added to the sample well, COVID-19 (SARS-CoV-2) will combine with the COVID-19 antibody conjugate to form an antigen-antibody complex. This complex migrates through nitrocel- lulose membrane by capillary action. When the complex meets the line of the COVID-19 antibody of test line T, the complex is trapped forming a burgundy colored band which confirms a reactive test result. Absence of a colored band in the test region indicates a non-reactive test result.
The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocom- plex goat anti-chicken IgY/chicken IgY-gold conjugate regardless of the color development on any of the test bands. Otherwise, the test result is invalid and the specimen must be retested with another device.
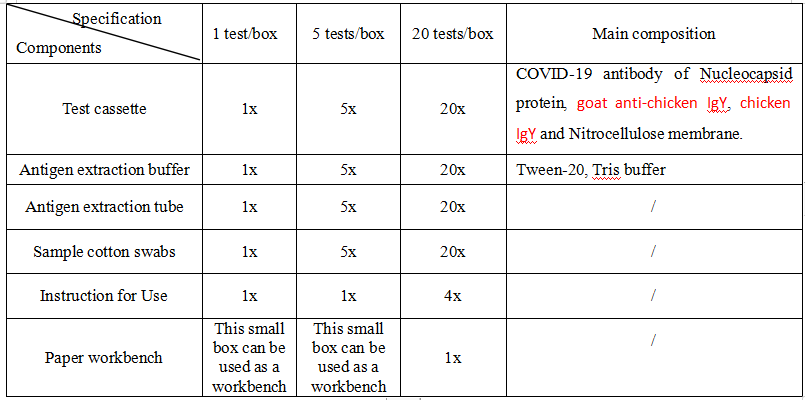
MATERIALS SUPPLIED
MATERIALS REQUIRED BUT NOT PROVIDED
Timer
STORAGE AND STABILITY
The kit can be stored at 4-30℃. The test device is stable through the expiration date printed on the sealed pouch. The test device must remain in the sealed pouch until use.
Do not freeze.
Do not use after expiry.
WARNINGS AND PRECAUTIONS
· Use the test kit once only. Do not reuse the test cassette or buffer.
· Do not use the kit if the pouch/tube is damaged or broken.
· Remove the test device from the sealed pouch only when you are ready to perform the test.
· Use only the components of this test kit.
· Inadequate or improper sample collection may lead to inaccurate or false results.
· Avoid contact with skin and eyes. In case of accidental contact, rinse well. In case of concerns, consult your doctor.
· Keep the test kit away from children to reduce the risk of accidentally drinking the buffer or swallowing small parts.
· This test is an aid for diagnosis.
· If your test result is positive, you should follow the guidance from your local State or Territory Health Department for guidance on confirmation testing if necessary, and if unwell seek medical assistance.
· Even if your test result is negative, continue to observe all applicable hygiene and safety measures. Even with a negative result, you may still be infectious.
· Repeat testing is recommended (e.g. within 1-3 days) if there is an ongoing suspicion of infection, being in a high risk setting or where is an occupational risk or other requirement.
LIMITATIONS
· The sample should be processed with antigen extraction buffer immediately after collection. Samples treated with antigen extraction buffer can be stored for 24 hours at room temperature (10℃-30℃), 48 hours at 2℃-8℃ degrees, and 15 days at -20℃ freezing. The sample should be frozen and thawed no more than 3 times. Use fresh samples whenever possible.
· Optimal assay performance requires strict adherence to the assay procedure described in this IFU. Deviations may lead to aberrant results.
· Negative results do not rule out a SARS‑CoV‑2 infection and should not be used as the sole basis for treatment or patient management decisions, including decisions about infection control. If symptoms persist, please follow the guidance from your local State or Territory Health Department for guidance on confirmation testing if necessary, and if unwell seek medical assistance.
· Only use for in vitro diagnostic, and cannot reused.
· Positive results do not exclude the possibility that a bacterial infection or a co‑infection with another virus is present.
· Positive results indicate the presence of viral antigens. However, a clinical correlation with the case history and other diagnostic information are required to determine the status of the infection.
· False negative test results (i.e., an existing infection is not detected) may occur if testing is not performed within the first 7 days of symptom onset as the antigen level in the specimen may be too low.
· False negative test results may occur if the specimen was collected incorrectly.
· False negative test results may occur if the specimen swab is not mixed well in the tube.
· False positive results may occur in the presence of SARS‑CoV infections.
· The test should be used for the detection of COVID-19 antigen in human nasal swab.
PERFORMANCE CHARACTERISTICS
1.Clinical Performance
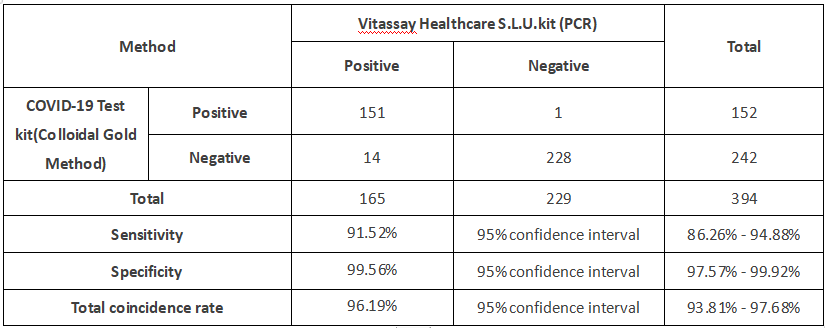
The results of the COVID-19 Test Kit (Colloidal Gold Method) were compared to results of RT-PCR assays for SARS-CoV-2 in nasal swab specimens. A total of 394 nasal swabs samples were tested in this study. 165 nasal swabs samples from RT-PCR confirmed SARS-CoV-2 positive cases (from early infection within the first 7 days after symptom onset). 229 nasal swabs samples from RT-PCR confirmed COVID-19 negative cases, including other respiratory pathogens with suspected symptoms. The sensitivity of the COVID-19 Test Kit (Colloidal Gold Method) showed a sensitivity of 91.52% and a specificity of 99.56% compared to PCR.
Usability Sutdy
During the testing of 247 participants, in the comparison of the test results of layman and professionals, the positive coincidence rate was 96.00%, the negative coincidence rate was 100%, and the total coincidence rate was 98.38%. This shows that the product has good usability and consistency.
There was another usability study enrolling 12 layman, and the usability evaluation was 100% from the aspects of operation standardization, IFU readability and result interpretation, indicating that the product has good usability.
2.Limit of Detection (LOD)
The limit of detection for COVID-19 Test Kit (Colloidal Gold Method) was determined to be 80 TCID50/mL using inactivated SARS-CoV-2 Virus.
3.Variants
Any COVID-19 variants (including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), Omicron (B.1.1.529), BA.4, BA.5, BA.2.75) ,are detected by this test without any impact on performance.
4.High Dose Hook Effect
No high dose hook effect was observed when testing up to a concentration of 1.6 x 107 TCID50/mL of heat inactivated SARS-CoV-2 virus.
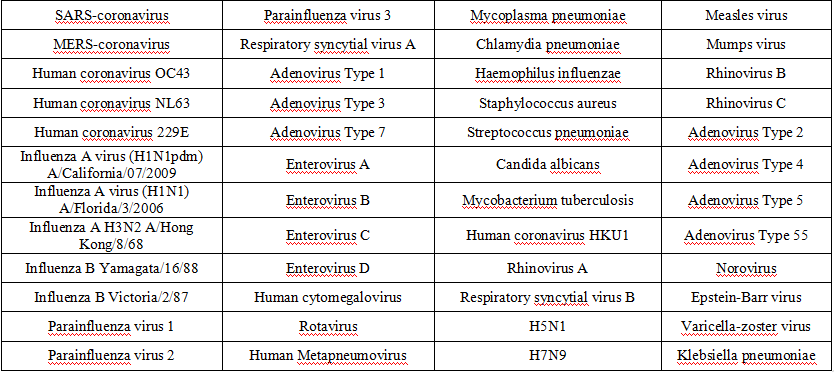
5.Cross Reactivity
The potential cross-reactivity of common organisms (refer to table below) was evaluated with SARS-CoV-2 negative and positive samples using the COVID-19 Test Kit (Colloidal Gold Method). The results showed no cross-reactivity.
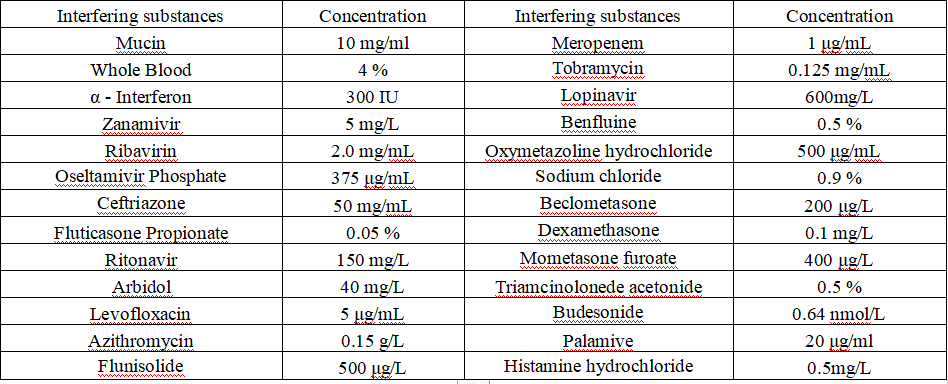
6.Interfering substance
The following potential interference substances were evaluated with the COVID-19 Test Kit (Colloidal Gold Method) and were found not to affect test performance.
TEST PROCEDURE
Before starting
Wash your hands with soap and water or use a hand sanitizer before performing the test. Make sure they are dry before starting.
1.Preparing for the test
1A. Check the expiration date on the box. Do not use if the kit has been damaged or has expired.
1B. Ensure that all test components are kept at room temperature (15-25℃) prior to use.
Open the box carefully as it will be used in a later step (1C).
Note: Keep a clock, timer or stopwatch at hand.
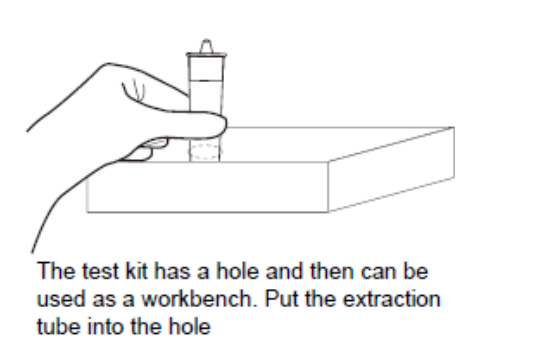
1C. Place the antigen extraction tube on the paper workbench and remove the dropper cap.
Note: This small box can be used as a workbench.
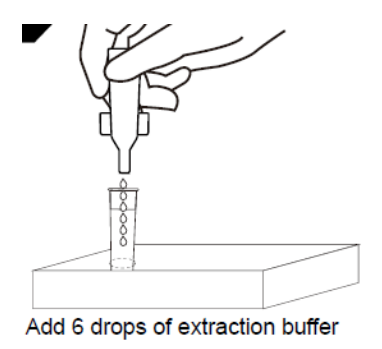
1D:Open the antigen extraction buffer bottle and place vertically downward, squeeze the bottle to make the buffer drip freely into the extraction tube without touching the edge of the tube, and add 6 drops (about 200μL) to the extraction tube.
2.Collecting and preparing a nasal sample
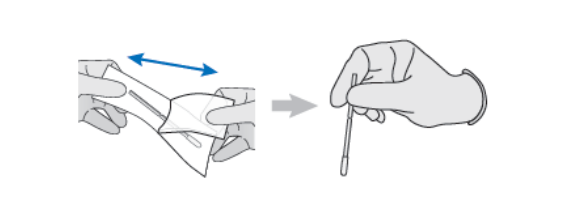
2A. Remove the swab from the packaging. Ensure that you only touch the handle of the swab and not the soft pad and the tip.
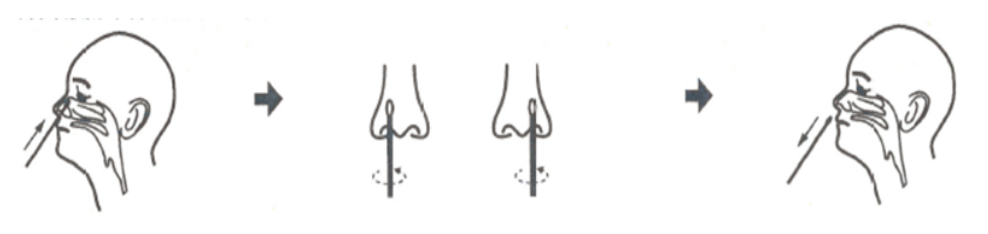
2B. Remove the secretions on the surface of the anterior nasal cavity, keep the head slightly tilted, hold the swab in one hand and stick the tail of the swab into one nostril, along the bottom of the lower nasal passage, and slowly down 1-1.5cm (for subjects aged 2-14 years,1 cm deep), and then stick it into the nasal cavity rotate at least 4 times (with a dwell time of no less than 15 seconds), then repeat the same operation for the other nasal cavity using the same swab.
2C. Insert the swab into an extraction tube pre-added with the antigen extraction buffer. The swab head is rotated in the antigen extraction buffer for at least 30 seconds, while the swab head is pressed by hand at least 5 times across the outer wall of the antigen extraction tube to ensure that the sample is fully eluted in the antigen extraction tube.
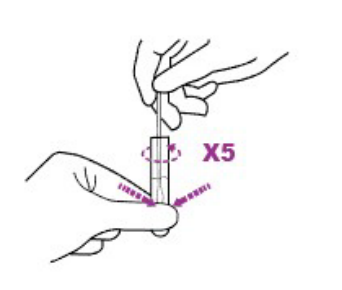
2D. Remove the swab while squeezing the tip of the swab to extract the liquid from the swab.
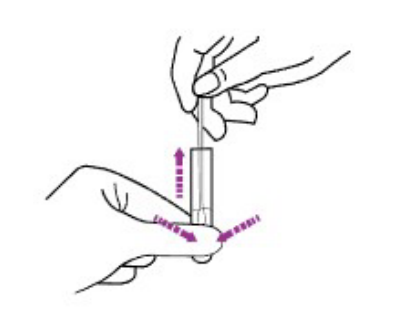
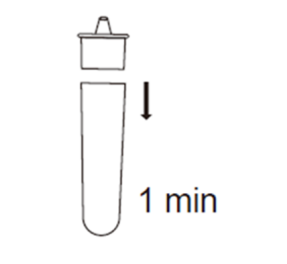
2E.Fit the dropper cap tightly onto the top of the extraction tube, and let it stand for about 1 minute.
3.Performing the test
3A. Remove the test cassette from the sealed foil pouch and use it within 1hour.
Note: Best results will be obtained if the test is performed immediately after opening the foil pouch.
Place the test cassette on a clean and level surface.
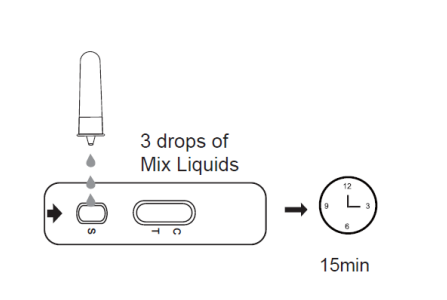
3B. Invert the antigen extraction tube (2E) and add 3 drops of extracted solution into the specimen well, and start the timer.
3C. Wait for the colored line to appear. The result should be read in 15 minutes. Do not interpret the result after 20 minutes.
4.Read test result
NEGATIVE:
One colored line appears at the control region(C), and no line appears at the test region (T).

A negative test result indicates that you are unlikely to infected with COVID-19.
If the test result is negative:
Continue to follow all applicable rules regarding contact with others and protective measures. Even if the test is negative, an infection may be present.
In case of suspicion, repeat the test after 1 - 2 days, because the Coronavirus cannot be accurately detected at all stages of infection.
COVID-19 Positive:
Two lines appear. One colored line appears at the control region(C), and another appears at the test region (T).
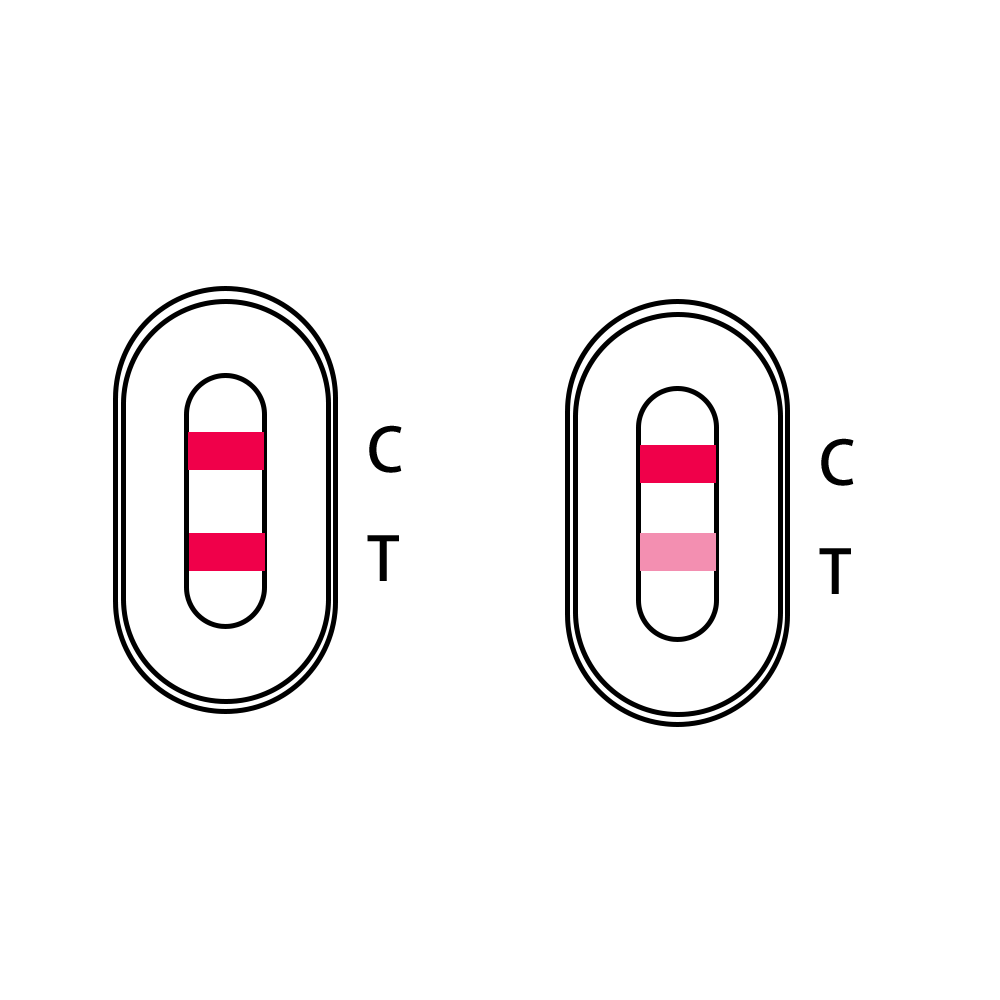

A positive test result indicates that you are likely to infected with COVID-19. You should follow the guidance from your local State or Territory Health Department for guidance on confirmation testing if necessary, and if unwell seek medical assistance.
INVALID:
Control line(C) fails to appear.

Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure.
In case of an invalid test result:
- Review the procedure and repeat the test with a new test cassette.
- If test results are still invalid, contact the sponsor.
5.Dispose of the used test kit
5A.Carefully wrap the used test kit components and swab samples and dispose in normal household waste.
5B. Wash your hands thoroughly after handling.


(Youtube Video for TEST PROCEDURE)
https://youtu.be/mixmIUp_070