CE COVID-19 Antigen Test kit (Colloidal Gold)
For Professional Use
CE
INTENDED USE
COVID-19 Antigen Test kit (Colloidal Gold) is a solid phase immunochromatographic assay for the rapid, qualitative detection of antigen to 2019 Novel Coronavirus in human saliva. This test provides only a preliminary test result. Therefore, any reactive specimen with the COVID-19 Antigen Test kit (Colloidal Gold) must be confirmed with alternative testing method (s) and clinical findings
PACK FORMATS
1test/box 20 tests/box 50 tests/box 100 tests/box
INTRODUCTION
The novel coronaviruses belong to the βgenus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection: asymptomatic only infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
PRINCIPLE
The COVID-19 Test Kit (Colloidal Gold Method) is a colloidal gold immunochromatographic assay. It detects the nucleocapsid protein on the surface of cOVID-19.
The test uses COVID-19(SARS-CoV-2) antibody (test line T) and goat ant-mouse lgG (control line C) immobilised on a nitrocellulose strip. The burgundy colored conjugate pad contains colloidal gold conjugated to another COVID-19 (SARS-Cov-2) antibody conjugated with colloid gold and mouse lgG-gold conjugates. When the processed buffer containing the sample is added to the sample well, COVID-19 (SARS-CoV-2) will combine with the COVID-19 antibody conjugate to form an antigen-antibody complex. This complex migrates through nitrocellulose membrane by capillary action. When the complex meets the line of the COVID-19 antibody of test line T, the complex is trapped forming a burgundy colored band which confirm a reactive test result. Absence of a colored band in the test region indicates a non-reactive test result.
The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex goat anti-mouse lgG/mouse lgG-gold conjugate regardless of the color development on any of the test bands. Otherwise, the test result is invalid and the specimen must be retested with another device.
MATERIALS SUPPLIED
Sealed pouches each containing a test cassette, a desiccant
Sampling cotton swabs (for nasopharyngeal sampling only)
Antigen extract buffer
Antigen extraction tube
Paper workbench (The small one-test-box can be used as a workbench)
Instruction for use
MATERIAL REQUIRED BUT NOT PROVIDED
1. Specimen collection containers
2. Timer
STORAGE AND STABILITY
The kit can be stored at room temperature or refrigerated (4-30°C). The test device is stable through the expiration date printed on the sealed pouch. The test device must remain in the sealed pouch until use.
Do not freeze.
Do not use beyond the expiration date.
WARNINGS AND PRECAUTIONS
1. For professional In Vitro diagnostic use only. Do not use after expiration date
2. This package insert must be read completely before performing the test. Failure to follow the insert gives inaccurate test results.
3. Do not use it if the tube/pouch is damaged or broken.
4. Test is for single use only. Do not re-use under any circumstances.
5. Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout testing and follow the standard procedures for proper disposal of specimens.
6. Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assayed.
7. Humidity and temperature can adversely affect results
8. Do not perform the test in a room with strong air flow, ie. electric fan or strong air-conditioning.
SPECIMEN COLLECTION
1. COVID-19 Test kit (Colloidal Gold Method) can be performed using nasopharyngeal sampling.
2. Testing should be performed immediately after specimen collection.
3. Bring specimens to room temperature prior to testing.
4. If specimens are to be shipped, they should be packed in compliance with local regulations covering the transportation of etiologic agents.
TEST PROCEDURE
Allow test cassette, specimen, and antigen extract buffer control to equilibrate to room temperature (15-30°C) prior to testing.
1. Remove the test cassette from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
2. Place the test device on a clean and level surface.
Test procedure:
1. Ask the patient to remove the secretions on the surface of the anterior nasal cavity, keep the head slightly tilted, and gently and slowly insert the swab through the nasal cavity to the nasopharynx. When resistance is encountered, it will reach the posterior nasopharynx, stay for a few seconds to absorb secretions, and gently rotate to remove the swab.

2. Place the antigen extraction tube on the workbench. Place the antigen extraction buffer bottle vertically downward squeeze the bottle to make the buffer drip freely into the extraction tube without touching the edge of the tube, and add 6 drops (about 200ul) to the extraction tube.
3. Put the swab specimen into the extraction tube pre-added with the antigen extraction buffer, and rotate the swab about 10 times while pressing the swab head against the tube wall to release the antigen in the swab, then let it stand for about 1 minute.
4. Remove the swab while squeezing the tip of the swab so that as much liquid in the swab can be discharged as possible. Dispose of used swabs in accordance with biohazard waste disposal methods.
5. Install the dripper on the extraction tube and cap it tightly, and let it stand for about 1 minute.
6. Open the aluminum foil bag and take out the test card, add 3 drops (about 100ul) into the sample hole of the test card (or use a pipette to add 100ul), and start the timer.
7. Wait for the colored line to appear. The result should be read in 15 minutes. Do not interpret the result after 20 minutes.
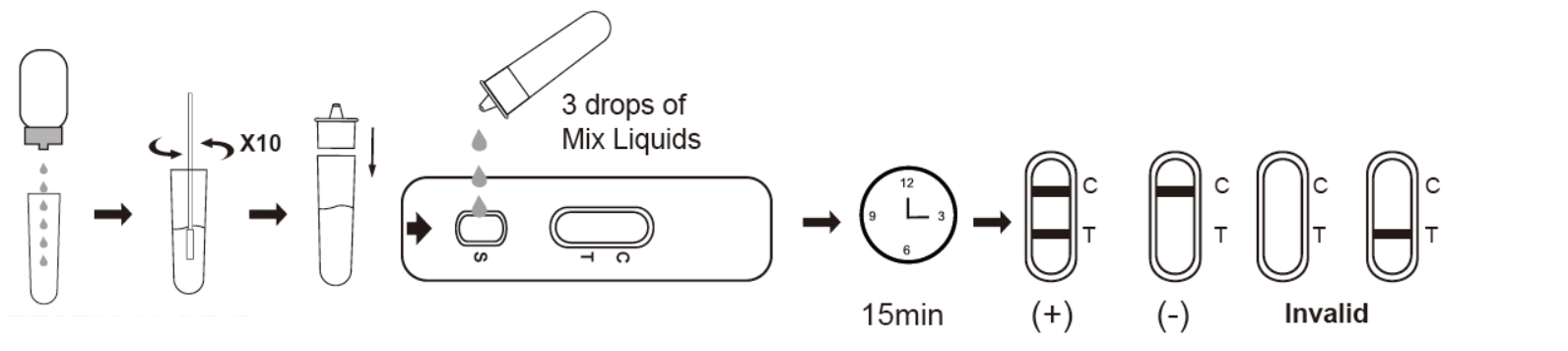
INTERPRETATION OF RESULTS
NEGATIVE:
If only the C band is present, the absence of any burgundy color in the T band indicates that no (COVID-19 SARS-CoV-2) antigen is detected in the specimen. The result is negative.
POSITIVE
In addition to the presence of C band, if T band is developed, the test indicates for the presence of (COVID-19 SARS-CoV-2) antigen in the specimen. The result is COVID-19 positive.
INVALID:
Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
LMITATIONS
1. Use fresh samples whenever possible.
2. Optimal assay performance requires strict adherence to the assay procedure described in this insert sheet. Deviations may lead to aberrant results.
3. A negative result for an individual subject indicates absence of detectable COVID-19 (SARS-CoV-2) antigen. However, a negative test result does not preclude the possibility of exposure to or infection with COVD-19.
4. A negative result can occur if the quantity of the COVID-19 (SARS-CoV-2) antigen present in the specimen is below the detection limits of the assay, or failed to collect the COVID-19 (SARS-COV-2) antigen in the nasal cavity of the patient.
5.As with all diagnostic tests, a definitive clinical diagnosis should not be based on the result of a single test, but should only be made by the physician after all clinical and laboratory findings have been evaluated.
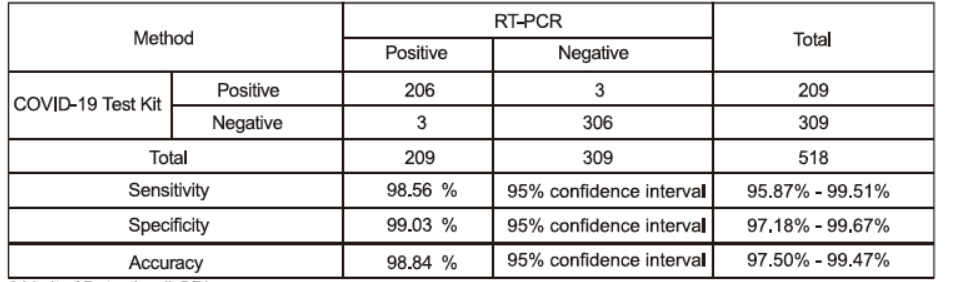
Clinical Sensitivity, Specificity and Accuracy
A total of 518 nasopharyngeal specimens were tested in this study, The COVID-19 clinical specimens contain specimens from individuals with symptoms within 7 days. The results of test reagent and control reagent both were 309 negative specimens and 209 positive specimens.
COVID-19 Test Kit vs PCR
Latest version 8.129.04.020 A10 lssued Date: 2023.01.03