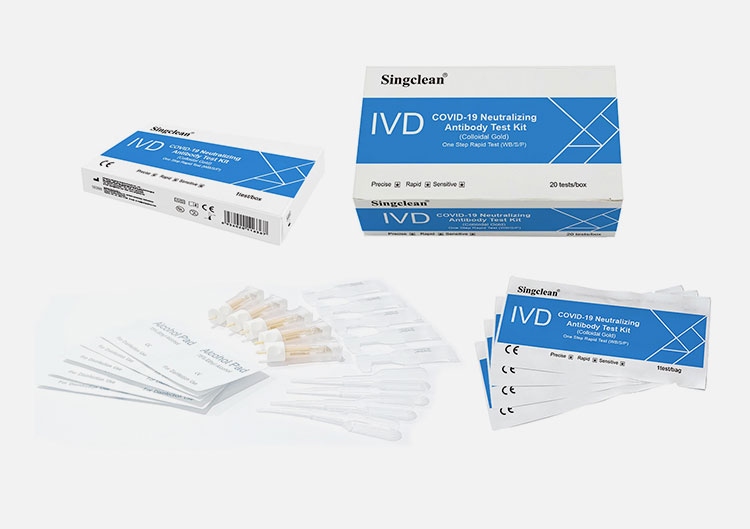
CE Singclean® COVID-19 Neutralizing Antibody Test Kit (Colloidal Gold)
CE
INTENDED USE
COVID-19 Neutralizing Antibody Test Kit (Colloidal Gold) is a solid phase immunochromatographic assay for the rapid ,qualitative detection of Neutralizing Antibody of COVID-19 in human whole blood/se-rum/plasma. This test provides only a preliminary test result.
PACK FORMATS
1 test/box, 20 tests/box,50 tests/box,100 tests/box
INTRODUCTION
The novel coronaviruses belong to the β genus.COVID-19 is an acute respiratory infectious disease. People are generally susceptible.
Infection with COVID-19 initiates an immune response, which includes the production of antibodies in the blood. The secreted antibodies provide protection against future viral infections, because they will stay inthe circulatory system from months to years after infection, and will quickly and strongly bind to thepathogen to block cellular infiltration and replication. These antibodies are named neutralizing antibodies.
PRINCIPLE
The COVID-19 Neutralizing Antibody Test kit (Colloidal Gold) is a colloidal gold immunochromatographic assay. Using the gene sequence of the COVID-19 virus surface spike protein S1 subunit (receptor binding domain, RBD), a RBD protein was produced; this RBD protein was labeled with colloidal gold, and the area of the detection line T of the nitrocellulose membrane was coated with a sufficient amount of Mouse anti-human polyclonal antibody, the new coronavirus neutralizing antibody will bind to the RBD protein and form a complex of RBD protein-neutralizing antibody-mouse anti-human polyclonal antibody in the area of the detection line T, making a purple-red line on the detection line T.
The test kit contains a quality control line (control line C), which should show a burgundy color band of goat anti-mouse polyclonal antibody combined with the colloidal gold conjugate in the gold label pad. regardless of the color on any other test line.
MATERIALS SUPPLIED
Sealed pouches each containing a test cassette, a desiccant
Dropper
Lancets (for finger stick whole blood only)
Sterilizing tablet (for finger stick whole blood only)
Buffer
lnstruction for use
MATERIAL REQUIRED BUT NOT PROVIDED
1.Specimen collection containers
2.Centrifuge (for plasma only)
3.Timer
STORAGE AND STABILITY
The kit can be stored at room temperature or refrigerated (4-30°C).The test device is stable through theexpiration date printed on the sealed pouch.The test device must remain in the sealed pouch until use.
DO NOT FREEZE.
Do not use beyond the expiration date.
After opening the sealed pouch, use the test as soon as possible within 60 minutes.
WARNINGS AND PRECAUTIONS
1. For professional In vitro diagnostic use only. Do not use after expiration date.
2. These instructions must be strictly followed by a trained healthcare professional to achieve accurate results. All users have to read the instruction prior to performing a test.
3. Do not use it if the tube/pouch is damaged or broken.
4. Wear protective gloves while handling specimens and wash hands thoroughly afterwards.
5. Avoid splashing or aerosol formation of specimen and buffer.
6. Clean up spills thoroughly using an appropriate disinfectant.
7. Decontaminate and dispose of all specimens, reaction kits and potentially contaminated materials (such as dropper, lancets, sterilizing tablet, test cassette) in a biohazard container as if they were infectious waste and dispose according to applicable local regulations.
8. Do not mix or interchange different specimens.
9. Do not mix reagent of different lots or those for other products.
10. Do not store the test kit in direct sunlight.
11. To avoid cross-contamination, do not reuse the dropper, lancets, sterilizing tablet and cassette.
12. Do not use with any solution except for the provided extraction buffer.
13. Test is for single use only. Do not re-use under any circumstances.
14. Do not perform the test in a room with strong air flow, such as electric fan or strong air-conditioning.
SPECIMEN COLLECTION
1. COVID-19 Neutralizing Antibody Test kit (Colloidal Gold) can be performed using whole blood/serum/plasma.
2. Testing should be performed immediately after specimen collection.
3. Bring specimens to room temperature prior to testing.
4. lf specimens are to be shipped, they should be packed in compliance with local regulations covering the transportation of etiologic agents.
TEST PROCEDURE
Allow test cassette, specimen, buffer and/or other materials to room temperature (15-30)℃ prior to testing.
1. Remove the test cassette from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within 60 minutes.
2. Place the test device on a clean and level surface.
For Serum or Plasma Specimens:
With the provided dropper, draw serum/plasma specimen and then add 1 drop (about 20ul) of serum/plasma specimen into the sample well(S). Then add 2 drops of buffer to the sample well(S) immediately. Avoid air bubbles.
For Whole Blood Specimen:
With the provided dropper and transfer 1 drop (about 20ul) of whole blood to the sample well(S) of the test device, then add 2 drops of buffer to the sample well(S) immediately. Avoid air bubbles.
3. Wait for the colored line(C) to appear. The result should be read after 10 minutes. Do not interpret the result after 20 minutes.
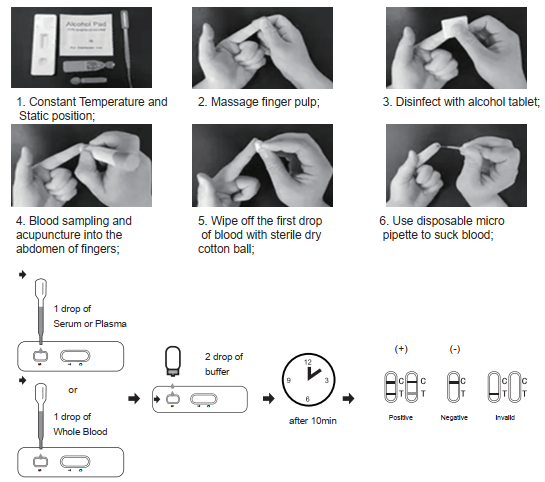
INTERPRETATION OF RESULTS
NEGATIVE:
lf only the C band is present, the absence of any burgundy color in the T band indicates that no COVID-19Neutralizing Antibody is detected in the specimen. The result is negative
POSITIVE:
In addition to the presence of C band, if T band is developed, the test indicates for the presence of COVID-19 Neutralizing Antibody in the specimen. The result is COVID-19 Neutralizing Antibody positive.
INVALID:
Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. lf the problem persists, discontinue using the test kit immediately and contact your local distributor.
LIMITATIONS
1. Use fresh specimen whenever possible.
2. Optimal assay performance requires strictly adherence to the assay procedure described in Instruction for use. Deviations may lead to aberrant results.
3. A positive test result indicates that there are COVID-19 neutralizing antibodies in the sample, and a positive reaction can be generated after infection with the COVID-19 or injection of the COVID-19 vaccine. It needs to be analyzed based on the individual situation of the user,
4. A positive COVID-19 neutralizing antibody test result only indicates that the tested person has neutralizing antibody of COVID-19 in the blood, but it does not mean that the tested person will be protected from the COVID-19 infection. Please pay attention to maintain personal protective measures in a crowded environment.
5.A negative result only indicates that there is no COVID-19 neutralizing antibody that reaches the test concentration in the specimen, and other cellular immunity and mucosal immunity cannot be ruled out.6.As with all diagnostic tests, a definitive clinical diagnosis should not be based on the results of a single test, but should be made by a doctor after evaluating the results of other clinical and laboratory tests.
Latest version 8.64.04.100 A2 lssued date: 2022-12-30