Malaria antigen test kit (Immunochromatography)
Instructions for Use
• For professional use only • For in vitro diagnostic use only
Please read this IFU carefully before use!
CE
[INTENDED USE]
The Malaria antigen test kit (Immunochromatography) is a rapid chromatographic immunoassay for the qualitative detection of circulating plasmodium falciparum (P.f.) and Pan-malaria antigens (P.f., P.v., P.o. and P.m.) in whole blood.
[INTRODUCTION]
Malaria is caused by a protozoan which invades human red blood cells Malaria is one of the world's most prevalent diseases According to the WHO, the worldwide prevalence of the disease is estimated to be 300-500 million cases and over 1 million deaths each year. Most of these victims are infants, young children. Over half of the world's population lives in malarious areas. Microscopic analysis of appropriately stained thick and thin blood smears has been the standard
diagnostic technique for identifying malaria infections for more than a century. The technique is capable of accurate and reliable diagnosis when performed by skilled microscopists using defined protocols. The skill of the microscopists and use of proven and defined procedures, frequently present the greatest obstades to fully achieving the potential accuracy of microscopic diagnosis. Although there is a logistical burden associated with performing a time-intensive, labor-intensive,
and equipment-intensive procedure such as diagnostic microscopy, it is the training required to establish and sustain competent performance of microscopy that poses the greatest difficulty in employing this diagnostic technology. The Malaria antigen test kit (Immunochromatography) is a rapid test to qualitatively detect the presence of P falciparum-specific-HRP and Pan-LDH (Pan). The test utilizes colloid gold conjugate to selectively detect P.f-specific and Pan-LDH (Pan)-specific antigens in whole blood.
[PRINCIPLE]
The Malaria P.f/Pan Antigen Rapid Test Device is a qualitative, membrane based immunoassay for the detection of P.f. and Pan antigens in whole blood. The membrane is pre-coated with anti- HRP-II antibodies and monoclonal antibody is pan to the lactate dehydrogenase of Plasmodium species. During testing the whole blood specimen reacts with the dye conjugate, which has been pre-coated on the test device. The mixture then migrates upward on the membrane by capillary action, reacts with anti-histidine-rich Protein I(HRP-II)antibodies on the membrane on P.f. test line region and with anti-pan-LDH antibodies on the membrane on Pan Line region. If the specimen contains HRP-II or plasmodium-specific Pan LDH or both, a colored line will appear in P.f line region or pan line region or two colored lines will appear in P.f. line region and Pan line region. The absence of the colored lines in P.f. line region or Pan line region indicates that the specimen does not contain HRP-II and/or plasmodium-specific Pan-LDH. To serve as a procedure control, a colored line will always appear in the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
[MATERIALS PROVIDED]
Test device
Buffer
Dropper
Lancets (for fingerstick whole blood only)
Sterilizing tablet (for fingerstick whole blood only)
Instructions for use
[MATERIALS REQUIRED BUT NOT PROVIDED]
Timer
Specimen collection containers
Pipette (optional)
[STORAGE AND STABILITY]
●Store in the sealed pouch at 4°C to 30°C. The validity period is 24 months.
●The test is stable through the expiration date printed on the sealed pouch.
●The test must remain in the sealed pouch until use.
●After opening the sealed pouch, use the test as soon as possible within 60 minutes.
●Do not freeze.
●Do not use beyond the expiration date.
[SPECIMEN COLLECTION AND STORAGE]
●This product can be performed using whole blood.
●Both Fingerstick Whole Blood and Venipuncture Whole Blood can be used.
●To collect Fingerstick Whole Blood specimens:
●Wash the patient’s hand with soap and warm water or clean with an alcohol swab. Allow to dry.
●Massage the hand without touching the puncture site by rubbing down the hand towards the fingertip of the middle or ring finger.
●Puncture the skin with a sterile lancet. Wipe away the first sign of blood.
●Gently rub the hand from wrist to palm to finger to form a rounded drop of blood over the puncture site.
●Testing should be performed immediately after the specimens have been collected. Do not leave the specimens at room temperature for prolonged periods. Whole blood collected by venipuncture should be stored at 2-8°C if the test is to be run within 1 day of collection. Do not freeze whole blood specimens. Fingerstick Whole blood collected by capillary tube should be tested immediately.
●Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Specimens should not be frozen and thawed repeatedly.
●If specimens are to be shipped, they should be packed in compliance with local regulations covering the transportation of etiologic agents.
[TEST PROCEDURE]
Allow the test, specimen, buffer and/or controls to reach room temperature (15-30°C) prior to testing.
1.Bring the pouch to room temperature before opening it. Remove the test Device from the sealed pouch and use it as soon as possible.
2.Place the Device on a clean and level surface.
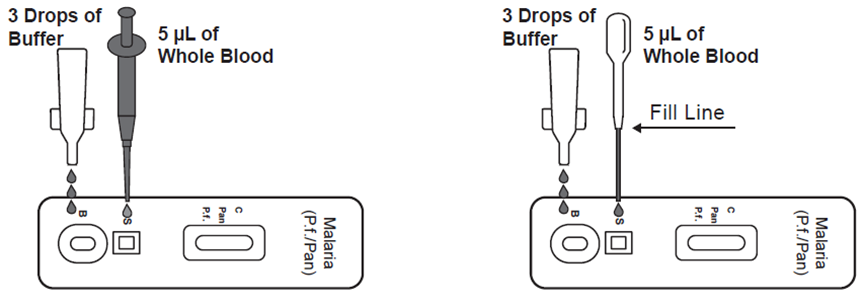
●Use a pipette: To transfer 5μL of whole blood to the specimen well (S), then add 3 drops of buffer to the buffer well (B) and start the time.
●Use a disposal specimen dropper: Hold the dropper vertically; draw the specimen up to the upper end of the nozzle as shown in illustration below (approximately 5μL). Transfer the specimen to the specimen well (S), then add 3 drops of buffer to the buffer well (B) and start the timer.
3.Wait for the colored line(s) to appear. Read results at 10 minutes. Do not interpret the result after 20 minutes.
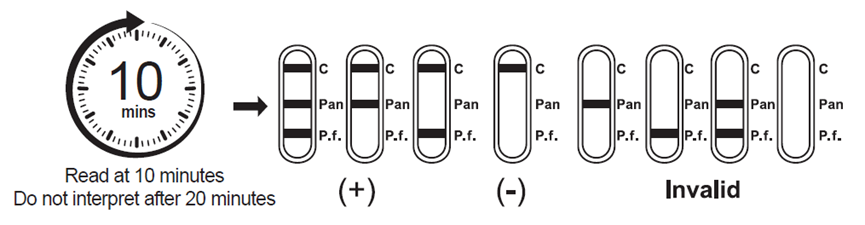
[INTERPRETATION OF RESULTS]
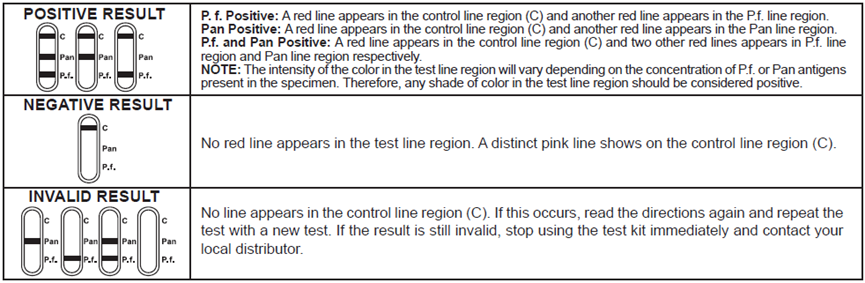
[LIMITATIONS]
1.This product is for in vitro diagnostic use only. This test should be used for the detection of P.f. and Pan antigens in whole blood specimens only. Neither the quantitative value nor the rate of increase in P.f. and Pan concentration can be determined by this qualitative test.
2.This product will only indicate the presence of antigens of Plasmodium sp. (P.f. and pan) in the specimen and should not be used as the sole criterion for the diagnosis of malaria infection.
3.As with all diagnostic tests, all results must be interpreted together with other clinical information available to the physician.
4.If the test result is negative and clinical symptoms persist, additional testing using other clinical methods is recommended. A negative result does not at any time preclude the possibility of malaria infection.
[PERFORMANCE CHARACTERISTICS]
1.Precision
The results of internal standard at the same concentration were consistent and the chromaticity was uniform.
2.Cross-reactivity
There was no cross-reactivity with the HAMA, RF, HBsAg, HBsAb, HBeAg, HBeAb, HBcAb, HCV, HIV, Syphilis, H.Pylori, CMV and Rubella.
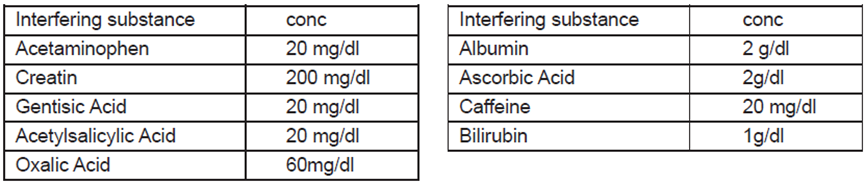
3.Interfering substance
The following potentially interfering substances were added to Malaria negative and positive controls. None of the substances at the concentration tested interfered in the assay.
[PRECAUTIONS]
●For professional in vitro diagnostic use only. Do not use after expiration date.
●Do not use if pouch is torn or damaged.
●For whole blood specimen use only. Do not use other specimens.
●Do not eat, drink or smoke in the area where the specimens or kits are handled.
●Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological
hazards throughout all procedures and follow the standard procedures for proper disposal of specimens.
●Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assayed.
●The used tests, specimens and potentially contaminated materials should be discarded according to the local regulations.
●Humidity and temperature can adversely affect results.
●Do not exchange or mix buffer and test devices from kits of different lot numbers.
●Caution must be taken at the time of specimen collection. Inadequate volume of specimen may lead to lower sensitivity.
●Be sure to add sufficient buffer to the Device's sample well. Invalid result may occur if inadequate buffer is added. For best
results, adhere to instructions provided.