Dengue Test Kit (Immunochromatography)
Instructions for Use
For in vitro diagnostic use
CE
[INTENDED USE]
The Dengue Test Kit (Immunochromatography) is a rapid chromatographic immunoassay for the qualitative detection of Dengue virus antigen in human whole blood, serum, or plasma. For professional use only.
[PACK FORMATS]
1 test/box, 5 tests/box, 10 tests/box, 20 tests/box
[INTRODUCTION]
Dengue viruses, transmitted by the mosquito, Aedes aegypti and Aedes albopictus mosquitoes, are widely distributed throughout the tropical and subtropical areas of the world. There are four known distinct serotypes (Dengue virus 1, 2, 3 and 4). In children, infection is often subclinical or causes a self-limited febrile disease. However, if the patient is infected second times with a different serotype, a more severe disease, Dengue hemorrhagic fever or Dengue shock syndrome, is more likely to occur. Dengue is considered to be the most important arthropod-borne viral disease due to the human morbidity and mortality it causes. Dengue is a highly-conserved glycoprotein that is present at high concentrations in the sera of Dengue-infected patients during the early clinical phase of the disease. antigen is found from the first day and up to 9 days after onset of fever in sample of primary or secondary Dengue infected patients.
[PRINCIPLE]
This kit is used for qualitative detection of Dengue virus antigen in human serum, plasma or whole blood by immunochromatography and double-antibody sandwich method. Monoclonal antibody against Dengue virus protein is coated on nitrocellulose membrane detection area (T). When the sample contains Dengue antigen and the concentration is higher than the minimum detection limit, Monoclonal antibodies against Dengue virus protein labeled with colloidal gold bind to Dengue virus antigen in serum, plasma, or whole blood to form a reaction complex, which moves forward along the nitrocellulose membrane by chromatography. It binds to monoclonal antibody of anti-Dengue antigen precoated in the test line (T) of nitrocellulose membrane to form a complex, and finally forms a visible red reaction line, at which time the result is positive. In contrast, when the sample does not contain Dengue antigen or the concentration is below the minimum detection limit, there is no red response line in the test area (T) and the result is negative.
The test kit contains a quality control line (control line C). A purple-red line appears on the control line regardless of the presence or absence of Dengue virus antigen in the sample. If the control line is not colored, it means that the test result is invalid regardless of whether the test line is colored. The control line is the criterion for judging whether the test strip is valid.
[MATERIALS PROVIDED]
1. Test cassette
2. Disposable pipettes
3. Buffer
4. Instruction for use
5. Lancets(for fingertip whole blood only)
6. sterilizing tablet (for fingerstick whole blood only)
[MATERIALS REQUIRED BUT NOT PROVIDED]
1. Specimen collection containers
2. Centrifuge (for plasma only)
3. Timer
[STORAGE AND STABILITY]
The kit can be stored at 4-30°C and should keep away from direct sunlight. Expiry after two years. Do not use beyond the expiration date. After opening the sealed pouch, use the test as soon as possible within 60 minutes.
[WARNINGS AND PRECAUTIONS]
1.This test provides only a preliminary screening Dengue test result. Therefore, any positive result must be confirmed with more accurate testing methods and clinical results.
2.Do not use after expiration date.
3.All users have to read the instruction prior to performing a test. These instructions must be strictly followed
4.Do not use it if the tube/pouch is damaged or broken.
5.Do not use if package is open or damaged.
6.Wear protective while handling specimens and wash hands thoroughly afterwards.
7.Avoid splashing or aerosol formation of specimen and buffer.
8.Clean up spills thoroughly using an appropriate disinfectant.
9.Decontaminate and dispose of all specimens, reaction kits and potentially contaminated materials in a biohazard container as if they were infectious waste and dispose according to applicable local regulations.
10.Do not mix or interchange different specimens.
11.Do not mix reagent of different lots or those for other products.
12.Do not store the test kit in direct sunlight.
13.Do not dilute the specimens with any solution except for the provided buffer.
14.Test is for single use only. Do not re-use under any circumstances.
15.Do not perform the test in a room with strong air flow.
16.In case of positive specimen, seek medical attention in time.
17.If the buffer touches eyes and mouth, a large amount of clean water should be rinsed in time and medical treatment should be sought if needed.
18.In case of any serious incident related to the device, it shall be reported to the manufacturer, distributor, EU authorized representative, notified body and the competent authority.
[SPECIMEN COLLECTION AND STORAGE]
1.The Test is intended for use with human whole blood, serum or plasma specimens only.
2.Separate the serum or plasma from the blood as soon as possible to avoid hemolysis. Use only clear, non-hemolytic samples.
3.To collect Fingerstick Whole Blood specimens:
●Wash the patient’s hand with soap and warm water or clean with an alcohol swab. Allow to dry.
●Massage the hand without touching the puncture site by rubbing down the hand towards the fingertip of the middle or ring finger.
●Puncture the skin with a sterile lancet. Wipe away the first sign of blood.
●Gently rub the hand from wrist to palm to finger to form a rounded drop of blood over the puncture site.
4.Testing should be performed immediately after the specimens have been collected. Do not leave the specimens at room temperature for prolonged periods. Serum and plasma specimens may be stored at 2~8°C for up to 7 days, for long termstorage, serum/plasma specimens should be kept below -20°C.Whole blood collected by venipuncture should be stored at 2~8°C if the test is to be run within 2 days of collection.
5.Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Specimens should not be frozen and thawed repeatedly.
6.If specimens are to be shipped, they should be packed in compliance with local regulations covering the transportation of etiologic agents.
[TEST PROCEDURE]
Allow test cassette, Antigen extraction buffer to equilibrate to room temperature prior to testing.
1.Open the aluminum foil bag and take out the test card.
2. Put the test card on a horizontal table, unscrew the top cap of the diluted sample, and slowly add 3 drops (about 100μL) of the pre-treated semen sample to the center of the sample hole;
3.Start timing, the reaction results should be observed within 10-15minutes, and the results are invalid after 15minutes.Allow test cassette, buffer to equilibrate to room temperature (15-30°C) prior to testing.
1. Remove the test cassette from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within 60 minutes.
2. Place the test device on a clean and level surface.
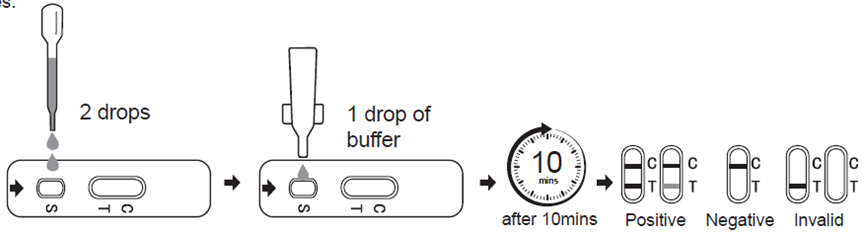
With the provided dropper, draw whole blood /serum/plasma specimen and then add 2 drop of serum/plasma specimen into the sample well(S). Then add 1 drops of buffer to the sample well(S) immediately. Avoid air bubbles.
3. Wait for the colored line(C) to appear. The result should be read after 10 minutes. Do not interpret the result after 15 minutes.
[INTERPRETATION OF RESULTS]
Positive (+) :
In addition to the presence of C band, if T band is developed, the test indicates for the presence of Dengue antigen in the specimen. The result is Dengue positive.
Negative (-) :
If only the C band is present, the absence of any burgundy color in the T band indicates that no Dengue antigen is detected in the specimen. The result is negative.
Invalid :
Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
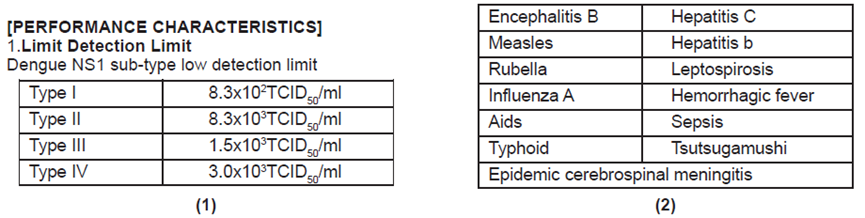
2.Cross Reaction
The following organisms were tested with The kit and has no effect on the negative and positive test results of this reagent, and there is no cross-reaction.
[LIMITATIONS]
1.The Dengue Test kit is intended for use with human whole blood, serum or plasma specimens only.
2.Use fresh samples whenever possible.
3.Only clear, non-hemolyzed specimens are recommended for use with this test. Serum or plasma should be separated as soon as possible to avoid hemolysis.
4.Whole blood collected by finger stick should be test immediately. Containers containing anticoagulants such as EDTA, citrate, or heparin should be used for whole blood storage.
5.Icteric, lipemic, hemolysed, heat treated and contaminated sera may cause erroneous results.
6.Optimal assay performance requires strictly adherence to the assay procedure described in Instruction for use. Deviations may lead to aberrant results.
7.A negative result for an individual subject indicates absence of detectable Dengue antigen.
8.A negative result can occur if the quantity of the Dengue antigen present in the specimen is below the detection limits of the assay.
9.However, a negative test result does not preclude the possibility of exposure to or infection with Dengue.
10.As with all diagnostic tests, a definitive clinical diagnosis should not be based on the result of a single test, but should only be made by the physician after all clinical and laboratory findings have been evaluated.
11.If specimens are to be shipped, pack them in compliance with all applicable regulations for transportation of etiological agents.
Version: 8.66.04.0006-A0 Issued date:2024-07-05