CE Monkeypox virus IgG/IgM Test Kit
(Immunochromatography)
CE
[Product name]
Monkeypox virus IgG/IgM Test kit (Immunochromatography)
[Packaging specifications]
1 test/box, 5 tests/box, 20 tests/box
[Intended use]
Monkeypox virus IgG/IgM Test Kit (Immunochromatography) is a solid phase immunochromatographic assay for the rapid, qualitative detection of IgG/IgM antibody to Monkeypox virus in human blood. This test provides only a preliminary test result. Therefore, any reactive specimen with the Monkeypox virus IgG/IgM Test Kit (Immunochromatography) must be confirmed with alternative testing method(s) and clinical findings.
[Test principle]
Monkeypox virus IgG/IgM Test kit(Immunochromatography) uses the principle of immunochromatography. The test uses anti-human IgM antibody (test line IgM), anti-human IgG (test line IgG) and goat anti-mouse IgG (control line C) immobilised on a nitrocellulose membrane. The conjugate pad contains microsphere conjugated to recombinant Monkeypox antigens conjugated with microsphere. When a specimen followed by assay buffer is added to the sample well, IgM &/or IgG antibodies if present, will bind to Monkeypox conjugates making antigen antibodies complex. This complex migrates through nitrocellulose membrane by capillary action. When the complex meets the line of the corresponding immobilized antibody (anti-human IgM &/or anit-human IgG) the complex is trapped forming a band which confirm a reactive test result.
Regardless of the presence of Monkeypox IgG/IgM antibody in the test sample, a colored band will appear on the quality control line (line C). The colored band appearing on the quality control line (line C) is used to determine whether there are enough samples and whether the chromatographic process meets the normal standard, and also serves as the internal control standard of the reagent.
[Materials Supplied]
Each sealed pouches contains a test cassette, a desiccant, a dropper, Buffer, Package insert
[Material required but not provided]
1. Specimen collection containers
2. Centrifuge
3. Timer
[Storage conditions and validity period]
The test kit should be stored in a sealed aluminum foil bag at 4℃~30℃, and the validity period is 24 months. After the foil bag is opened, the validity period is 1 hour.
[Sample requirements]
1. Human serum, plasma or whole blood samples; other body fluids and samples may not give accurate results.
2. Anticoagulation with EDTA, sodium citrate or heparin is recommended for plasma and whole blood samples.
3. Separate serum or plasma from blood as soon as possible to avoid hemolysis.
4. Testing should be performed immediately after specimen collection. After the clinical blood samples are collected, the test must be completed within 4 hours at room temperature; serum and plasma can be stored at 2~8°C for 3 days. For long term storage, specimens should be kept below -20°C. Whole blood samples should not be frozen and stored at 2~8°C for 3 days. Avoid heat inactivating samples, and hemolyzed samples should be discarded.
5. Samples must be returned to room temperature before testing. Refrigerated samples need to be completely thawed, rewarmed, and evenly mixed before use. Do not freeze and thaw repeatedly.
[Test method]
Allow test cassette, specimen, buffer and/or controls to equilibrate to room temperature prior to testing.
1. Remove the test cassette from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
2. Place the test device on a clean and level surface.
For Serum or Plasma Specimens:
With the provided dropper, draw serum/plasma specimen and then add 1 drop serum/plasma specimen into the sample well(S). Then add 2 drops of buffer to the sample well(S) immediately. Avoid air bubbles.
For Whole Blood Specimen:
With the provided dropper and transfer 1 drop of whole blood to the sample well(S) of the test device, then add 2 drops of buffer to the sample well(S) immediately. Avoid air bubbles.
Wait for the colored line(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 15 minutes.
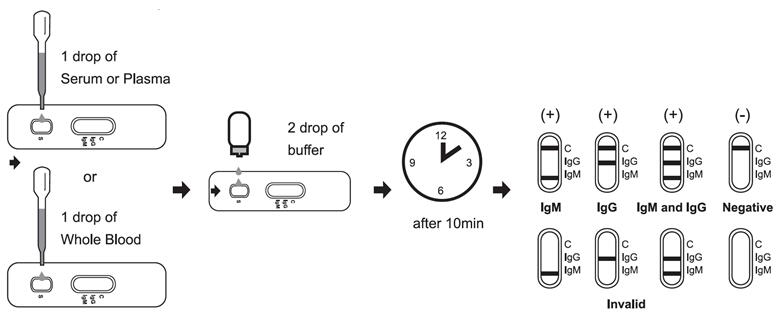
[Interpretation of results]
NEGATIVE: If only the C band is present, the absence of any burgundy color in the both T bands (IgG and IgM) indicates that no anti-Monkeypox antibodies are detected in the specimen. The result is negative.
IgM POSITIVE:
In addition to the presence of C band, if only IgM band is developed, the test indicates for the presence of IgM anti-Monkeypox in the specimen. The result is IgM anti-Monkeypox positive.
IgG POSITIVE:
In addition to the presence of C band, if only IgG band is developed, the test indicates for the presence of IgG anti-Monkeypox in the specimen. The result is IgG anti-Monkeypox positive.
IgG and IgM POSITIVE:
In addition to the presence of C band, both IgG and IgM bands are developed, the test indicates for the presence of both IgG and IgM anti-Monkeypox in the specimen. The result is IgG and IgM anti-Monkeypox positive.
INVALID:
Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
[Quality control]
A procedural control is included in the test. A red line appearing in the control region (C) is the internal procedural control. It confirms sufficient specimen volume and correct procedural technique.
Control standards are not supplied with this kit; however it is recommended that positive and negative controls be tested as a good laboratory practice to confirm the test procedure and to verify proper test performance
[Limitations of the test method]
1. This test kit is only for the test of human serum, plasma or whole blood samples. Abnormal hematocrit samples have an impact on the results of the whole blood test.
2. The test results need to be combined with other clinical and laboratory data, and if the Monkeypox IgG/IgM test results do not match the clinical assessment, further testing is required.
3. False positive results may be caused by: cross-reaction of antibody-like components in the blood; some nonspecific components in blood have similar antigenic determinants to capture antibodies.
4. False negative results may be caused by: some unknown components shield the antigenic determinants from binding to the antibody; the unstable Monkeypox antigen gradually degrades with time and temperature and cannot be recognized by the antibody. Effective test results depend on good reagent and sample storage environment.
5. Other factors, including technical reasons, operational errors and other sample factors, may also cause errors in test results.
6. Recent or remote vaccination with a vaccinia-based vaccine (e.g. anyone vaccinated before smallpox eradication, or more recently vaccinated due to higher risk such as orthopoxvirus laboratory personnel) might lead to false positive results.
7. Do not use if package is open or damaged.
[Warning]
1. For in vitro use only.
2. For professional use only.
3. Test is for single use only. Do not re-use under any circumstance.
4. All specimens should be considered potentially hazardous and handle in the same way as an infectious material. Wear protective clothing such as laboratory coats, disposable gloves.
5. The used testing materials should be discarded in accordance with local, state and or federal regulations.
6. In case of any serious incident related to the device, it shall be reported to the manufacturer, distributor, EU authorised representative, notified body and the competent authority.
Latest Version:8.75.04.004 A1 Issued Date: 2023.01.04