HIV-1/2 antibody urine assay kit (Colloidal gold)
[Product Name]
HIV-1/2 antibody urine test kit (Colloidal Gold Method)
[Packaging Specifications]
Cassette: 1 test/box, 10 tests/box, 20 tests/box, 50 tests/box.
[Intended Use]
This product is used for the qualitative determination of human immunodeficiency virus type I (HIV-1) antibodies and type II (HIV-2) antibodies in human urine samples. It is suitable for the auxiliary diagnosis of HIV infection. The test results are only for clinical reference and cannot be used alone as the basis for confirming or excluding cases. In order to achieve the purpose of diagnosis, the test results should be used in conjunction with clinical examination, medical history and other examinations. This product can be used for consumer self-test.
[Test Principle]
HIV-1/2 antibodies are found in the urine of AIDS patients. This product uses the technology of immunochromatography to qualitatively detect HIV-1/2 antibodies in human urine samples. The urine sample is dripped into the sample well and chromatographed under the capillary effect. If there is HIV-1/2 antibody in the sample, the HIV-1/2 antibody in the sample will combine with gold-labeled HIV recombinant antigen to form a gold-labeled complex. The complex continued to move forward and specifically bound to the HIV-1/2-coated recombinant antigen of the detection line, and a purple-red line appeared. If there is no HIV-1/2 antibody in the sample, the gold-labeled complex will not be formed, and it will not specifically bind to the HIV-1/2-coated recombinant antigen immobilized at the detection line during the chromatography process, so that it can be detected during the detection process. The line is not colored;
A purple-red line appears on the control line regardless of the presence or absence of HIV-1/2 antibodies in the sample. If the control line is not colored, it means that the test result is invalid regardless of whether the test line is colored. The control line is the criterion for judging whether the test strip is valid.
[Main Components]
1. Product: It consists of test card, instruction manual, desiccant and dropper.
2. Test card: composed of nitrocellulose membrane coated with HIV-Ⅰ recombinant antigen, HIV-Ⅱ recombinant antigen and goat anti-mouse polyclonal antibody, gold pad coupled with HIV recombinant antigen with colloidal gold, specially treated glass fiber , filter paper and plastic plate cards and other components.
[MATERIALS REQUIRED BUT NOT PROVIDED]
1. Specimen collection containers
2. Centrifuge
3. Timer
[Storage Conditions and Validity Period]
1. Store at 4~30℃, avoid light and dry, valid for 24 months.
2. See the outer packaging for the production date and expiration date. Do not freeze or use after expiration date.
3. After opening the sealed pouch, use the test as soon as possible within 1 hour.
[Sample requirements]
1. Collect urine sample
2. When sampling, make sure the urine cup is clean to avoid contaminating the sample.
3. The sample should be clarified. If there is visible precipitation, it should be filtered, centrifuged or the supernatant should be collected after precipitation.
4. Keep the urine sample as fresh as possible. If not used temporarily, the sample can be stored at 2-8 degrees for one month.
5. If specimens are to be transported, they should be packaged in accordance with local regulations regarding the transport of pathogens.
[Testing method]
Allow the test box to equilibrate to room temperature prior to testing.
1. Remove the test card from the sealed foil bag and use it as soon as possible. After opening the sealed pouch, use the test as soon as possible within 1 hour.
2. Place the test apparatus on a clean, level surface.
3. Aspirate the urine sample with the provided dropper, then add 3 drops (approximately 100ul) of urine sample to the sample well. Start the timer.
4. Waiting for results. Results should be read after 15 minutes. The results after 20 minutes are not convincing.
[Explanation of test results]
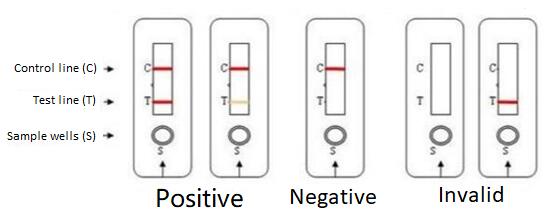
Negative:
If only the C line is present and there is no purple-red in the T line, HIV-1/2 antibodies are not detected in the specimen. The result was negative.
Positive:
In addition to the presence of a C-line, if a T-line is present, the test indicates the presence of HIV-1/2 antibodies in the sample. The result was positive.
Invalid:
Control lines do not appear. Regardless of whether there is a T line, it is an invalid result.
[Performance]
1. Minimum detection amount
The minimum detection limit was 0.2NCU/ mL using a constant reference diluted by negative urine
2. The sensitivity
The positive detection rate was 100% for 200 hiv-1 antibody positive serum samples diluted 10-fold with normal urine, and 100% for 50 HIV-2 positive reference samples diluted 10-fold with normal urine.
3. specificity
361 urine samples were negative, and the negative coincidence rate was 96.2%
4. Precision
Tested continuously for 20 days, the precision was consistent between batches and within batches, and the coincidence rate of repeated test was 100% for the same samples.
5. Cross reaction
Syphilis,hepatitis c, hepatitis B,CEA,Flu A , Flu B , HAMA , RF , ALT , COVLD-19,urine occult blood, total protein, urinary bilirubin no cross reaction in positive samples with the kit.
6. interfering material
MOP THC COC MET OPI BZO TCA OXY AMP MTD BAR MDMA no interference at 200mg/ml.
zidovudine Edurant nevirapine ritonavir famciclovir isoniazid no interference at 100mg/ml
7. HIV subtypes detected
It can detect HIV-1 type A, B, C, E, O . Also can detect HIV-2.
[Limitations of the test method]
1. This kit is a clinical auxiliary diagnostic product, which can rapidly detect human immunodeficiency virus type I (HIV-1) antibody and type II (HIV-2) antibody in urine. The test results are for clinical reference only and cannot be used alone as the basis for confirming or excluding cases. For the purpose of diagnosis, the test results should be used in conjunction with clinical examination, medical history and other examinations.
2. Due to the limitations of the method, a negative test result does not rule out the possibility of HIV infection in patients, especially those infected in the window phase.
3. Samples undergoing antiviral infection treatment may have false negatives.
4. The test sample is urine, and other body fluids may be inaccurate 。
5. The presence of interfering substances in the sample may lead to incorrect test results.
[Warning]
1. For in vitro use only.
2. For single use. Do not use after expiration date.
3. Use fresh Urine samples whenever possible.
4. This product can be for professional use. Also can be for self-testing.
5. The used testing materials should be discarded in accordance with local, state and or federal regulations.
6. In case of any serious incident related to the device, it shall be reported to the manufacturer, distributor, EU authorised representative, notified body and the competent authority.
7. Interpretation of self-testing results and follow-up processing methods.
7.1 Negative test result: Indicates no infection, only in rare cases, a positive sample will be tested as negative. First, confirm that the operation is correct, and then comprehensively consider whether there is a high-risk behavior. If there is a high-risk behavior recently, the "window phase" cannot be ruled out, and follow-up irregular testing is required.
7.2 Positive test results: suggest possible infection. First, confirm that the operation is correct, and you should test again; if the test is still positive, please go to the hospital, CDC and other medical and health institutions for confirmatory testing as soon as possible.
7.3. Invalid test result: The prompt result is invalid. First, confirm that the operation is correct and test again; if the test is still invalid, you need to change the test method or go to a medical and health institution for testing.
8. All specimens should be considered potentially hazardous and handle in the same way as an infectious material.
Latest Version 8.58.04.002 A0 lssued Date: 2022-06-10