HBsAg Test kit (Colloidal Gold)
[Product name]
HBsAg Test Kit (Colloidal Gold)
[Packaging specifications]
Strip: 50 tests/box Cassette: 20 tests/box
[Intended use]
HBsAg Test kit (Colloidal Gold) is used for qualitative detection of Hepatitis B surface Antigen in human serum or plasma samples. Hepatitis B surface Antigen (HBsAg) is the hepatitis b virus coat protein. It's not contagious, only the antigenicity. But it is usually accompanied by the presence of hepatitis b virus, so it is a sign of the hepatitis b virus infection.
[Test principle]
HBsAg Test kit (Colloidal Gold) uses colloidal gold immune chromatography technology to qualitative detection of Hepatitis B surface Antigen in human serum or plasma samples.
The sample is dripped into the sample well and chromatographed under the capillary action. If there is HBsAg in the sample, the HBsAg in the sample will combine with gold-labeled HBsAg recombinant antibody to form a gold-labeled complex. The complex continued to move forward and specifically bound to another HBsAg recombinant antibody of the detection line, and a purple-red line appeared. lf there is no HBsAg in the sample, the gold-labeled complex will not be formed, and it will not specifically bind to another HBsAg recombinant antibody immobilized at the detection line during the chromatography process, so that it can be detected during the detection process. The line is not colored.
A purple-red line appears on the control line regardless of the presence or absence of HBsAg in the sample. If the control line is not colored, it means that the test result is invalid regardless of whether the test line is colored. The control line is the criterion for judging whether the test strip is valid.
[Materials Supplied]
1. Test kit strip or test kit cassette2.Desiccant
3. Instruction for use
[Material required but not provided]
1. Specimen collection containers
2. Timer
3. Centrifuge
[Storage conditions and validity period]
1. Store at 4~30 ℃ , avoid light and dry, valid for 24 months.
2. See the outer packaging for the production date and expiration date. Do not freeze or use after expiration date.
3. After opening the sealed pouch, use the test as soon as possible within 1 hour.[Sample requirements]
1. Serum and plasma can be used for testing. The commonly used anticoagulants (EDTA, heparin and sodium citrate) in clinic did not affect the test results of plasma samples,
2. Use fresh samples whenever possible.
3. The collection of venous serum and plasma should be under sterile conditions, should avoid sample hemolysis.
4.If serum or plasma samples are tested within 7 days after collection, the samples must be stored at 2-8 ℃C or frozen if greater than 7 days.
[Test method]
Allow the test box to equilibrate to room temperature prior to testing. Test cassette
1. Remove the test card from the sealed foil bag and use it as soon as possible. After opening the sealed pouch, use the test as soon as possible within 1 hour.
2. Place the test apparatus on a clean, level surface.
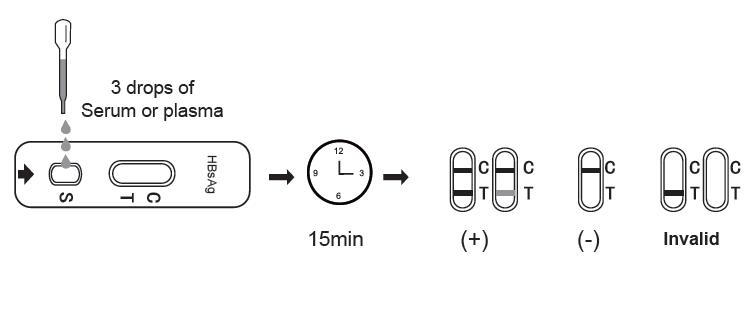
3. Aspirate the serum/plasma sample using the provided pipette, and add 3 drops (approximately 100 μL) of the sample to the sample well. Start the timer.
4. Wait for the colored line(s) to appear. The result should be read after 15 minutes. Do not interpret the result after 20 minutes.
Test strip
1. Remove the test strip from the sealed foil bag and use it as soon as possible. After opening the sealed pouch, use the test as soon as possible within 1 hour,
2. Dip the reagent strip into the sample, the sample level is within the water ripple range, immerse for15-20 seconds, flat on the table.
3. Waiting for results. Results should be read after 15 minutes. The results after 20 minutes are not convincing.
[Interpretation of results]
NEGATIVE:
If only the C band is present, the absence of any burgundy color in the T band indicates that no HBsAg is detected in the specimen. The result is HBsAg negative.
POSITIVE:
In addition to the presence of C band, if T band is developed, the test indicates for the presence of HBsAg in the specimen. The result is HBsAg positive.
INVALID:
Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
[Performance]
According to the research on the usage and dosage of the product:
1. Limit of detection
The limit detection of adr, adw and ay subtypes were 5IU/ml.
The limit detection of recombinant antigen was 2.5 ng/ml.
2. Specificity
2.1 Cross reaction
Syphilis, hepatitis C,HIV, HAMA, carcinoembryonic antigen,RF,ALT, mononucleosis, influenza A virus culture medium, influenza B virus culture medium, novel coronavirus culture medium has no cross effect on this product.
2.2 Interference reaction
Ascorbic acid, zidovudine, hemoglobin, aspirin, lamivudine, bilirubin, ibuprofen, abacavir,triglycerides, oxymetazoline hydrochloride, tenofovir, serum albumin, antifungal drugs, emtricitabine, Methylamphet-amine, Elvitegravir, tenofovir alafenamide, morphine, abbovetide, raltegravir, dolutegravir. At concentration of 100μg/ml without interference.
[WARNINGS AND PRECAUTIONS]
1. For professional In Vitro diagnostic use. Do not use after expiration date.
2. This test kit is only for the test of human serum, plasma.
3. These instructions must be strictly followed by a trained healthcare professional to achieve accurate results. All users have to read the instruction prior to performing a test.
4. A negative result can occur if the quantity of the HBsAg antigen present in the specimen is below the detection limits of the assay.
5. As with all diagnostic tests, a definitive clinical diagnosis should not be based on the result of a single test, but should only be made by the physician after all clinical and laboratory findings have been evaluated.
6. Do not use it if the pouch or package is damaged or broken.
7. Wear protective while handling specimens and wash hands thoroughly afterwards.
8. Clean up spills thoroughly using an appropriate disinfectant.
9.Decontaminate and dispose of all specimens, reaction kit and potentially contaminated materials in a biohazard container as if they were infectious waste and dispose according to applicable local regulations.
10. Do not mix or interchange different specimens.11.Do not store the test kit in direct sunlight.
12. Test is for single use only. Do not re-use under any circumstances.
13. Do not perform the test in a room with strong air flow, i.e. electric fan or strong air-conditioning.
14. In case of positive samples, seek medical attention in time.
15. In case of any serious incident related to the device, it shall be reported to the manufacturer, distributor or the competent authority.
Latest version 8.54.04.002-A0 lssued Date: 2022.12.28