Singderm® Silk Mono-phasic Dermal Filler
Caution: It restricts this device to sale by or on the order of a licensed physician or properly licensed practitioner.
BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY.
1.DEVICE DESCRIPTION
Singderm® Silk injectable gel is a sterile, biodegradable, non-pyrogenic, viscoelastic, clear, colorless, homogeneous gel implant. it consists of cross-linked hyaluronic acid (HA) produced by Streptococcus species of bacteria, formulated to a concentration of 24 mg/ml and 0.3% w/v lidocaine in a physiologic buffer.
2.INTENDED USE/INDICATIONS
Singderm® Silk injectable gel is indicated for injection into the lips for lip augmentation and for correction of perioral rhytids in adults over the age of 18.
3.CONTRAINDICATIONS
·Singderm® Silk is contraindicated for patients with severe allergies manifested by a history of anaphylaxis or history or presence of multiple severe allergies.
·Singderm® Silk contains trace amounts of Gram-positive bacterial proteins and is contraindicated for patients with a history of allergies to such material.
·Singderm® Silk contains lidocaine and is contraindicated for patients with a history of allergies to such material.
4.WARNINGS
·The product must not be injected into blood vessels. Introduction of Singderm® Silk injectable gel into the vasculature may lead to embolization, occlusion of the vessels, ischemia, or infarction. Take extra care when injecting soft-tissue fillers, for example, after insertion of the needle, and just before injection, the plunger rod can be withdrawn slightly to aspirate and verify the needle is not intravascular, inject the product slowly, and apply the least amount of pressure necessary. Rare, but serious, adverse events associated with the intravascular injection of soft-tissue fillers in the face have been reported and include temporary or permanent vision impairment, blindness, cerebral ischemia or cerebral hemorrhage leading to stroke, skin necrosis, and damage to underlying facial structures. immediately stop the injection if a patient exhibits any of the following symptoms, including changes in vision, signs of stroke, blanching of the skin, or unusual pain during or shortly after the procedure. Patients should receive prompt medical attention and possibly evaluation by an appropriate health care professional specialist should an intravascular injection occur.
·Product use at specific sites in which an active inflammatory process (skin eruptions such as cysts, pimples, rashes, or hives) or infection is present should be deferred until the underlying process has been controlled.
· Injection site responses consist mainly of short-term inflammatory symptoms starting early after treatment and lasting ≤ 7 days.
5.PREGAUTIONS
· Singderm® Silk injectable gel is packaged for single-patient use. Do not resterilize. Do not use if package is opened or damaged.
In order to minimize the risks of potential complications, this product should only be used by health care professionals who have appropriate training, experience, and who are knowledgeable about the anatomy at and around the site of injection. Health care professionals are encouraged to discuss all potential risks of soft-tissue injection with their patients prior to treatment and ensure that patients are aware of signs and symptoms of potential complications.
Generally, patients should be limited to 20 mL of any Singderm® Silk injectable gel per 60 kg (130 lbs) body mass per year. The safety of injecting greater amounts has not been established.
·The safety and effectiveness for the treatment of anatomic regions other than the lips and perioral area have not been established.
As with all transcutaneous procedures, dermal filler implantation carries a risk of infection. Standard precautions associated with injectable materials should be followed.
·Singderm® Silk injectable gel is to be used as supplied. Modification or use of the product outside the Directions for Use may adversely impact the sterility, homogeneity, and performance of the product.
·The safety for use during pregnancy, in breast feeding females, or in patients under 18 years old has not been established.
The safety in patients with known susceptibility to keloid formation, hypertrophic scarring, and pigmentation disorders has not been studied.
·Singderm® Silk injectable gel should be used with caution in patients on immunosuppressive therapy.
·Patients who are using substances that can prolong bleeding (such as aspirin, nonsteroidal anti-inflammatory drugs, and warfarin) may, as with any injection, experience increased bruising or bleeding at injection sites.
·After use, treatment syringes and needles are biohazards. Handle and dispose of these items in accordance with accepted medical practice and applicable local, state, and federal requirements. Singderm® Silk injectable gel is a clear, colorless gel without visible particulates. In the event that the content of a syringe shows signs of separation and/or appears cloudy, do not use the syringe; notify Singclean Product Support at 86-571-6343-1868.
· if laser treatment, chemical peeling, or any other procedure. based on active dermal response is considered after treatment with Singderm® Silk, there is a possible risk of eliciting an inflammatory reaction at the implant site. An inflammatory reaction is also possible if the product is administered before the skin has healed completely after such a procedure.
·Failure to comply with , the needle attachment instructions could result in needle disengagement and/or product leakage at the LUER-LOK® and needle hub connection.
6.UNDESIRABLE EFFECTS
The patients must be informed that they are potential side effects associated with implantation of this product, which may occur immediately or may be delayed. These include, but are not limited to swelling, tenderness, firmness, bruising, lumps/bumps, redness, pain, discoloration, itching, and dryness. Few treatment-related AEs. after initial treatment (or touch-up treatment) included chapped lips, dizziness, dry lips, general physical condition abnormal, headache, lip disorder (lumps), lip injury, oral herpes, presyncope, wound, and injection site discoloration, discomfort, edema, erythema, exfoliation, hyperaesthesia, hypoaesthesia, laceration, nodule, papule, paraesthesia, pruritus, and reaction. Most of the symptoms resolved without any treatment.
Patients must report reactions which persist for more than one week, or any other side effect which develops, to their medical practitioner as soon as possible.
7.INSTRUCTIONS FOR USE
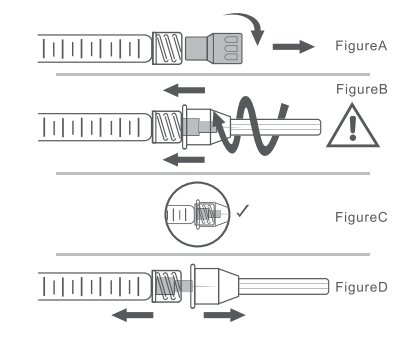
A. To Attach Needle to Syringe
STEP 1: Remove tip cap
Hold syringe and pull tip cap off the syringe as shown in Figure A.
STEP 2: Insert needle
Hold the syringe body and firmly insert the hub of the needle (provided in the Singderm® Silk package) into the LUER-LOK® end of the syringe.
STEP 3: Tighten the needle
Tighten the needle by turning it firmly in a clockwise direction (see Figure B) until it is seated in the proper position as shown in Figure C.
NOTE: If the position of the needle cap is as shown in Figure D, it is not attached correctly. Continue to tighten until the needle is seated in the proper position.
STEP 4: Remove the needle cap
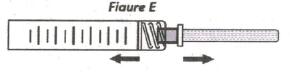
Hold the syringe body in one hand and the needle cap in the other. Without twisting, pull in opposite directions to remove the needle cap as shown in Figure E.
B. Health Care Professional Instructions
1. Singderm® Silk injectable gel is a cross-linked, soft, smooth gel formulation that can be injected using a fine gauge (e.g., 30G) needle into the lips and perioral area to add fullness and improve the shape of the lips, and to smooth perioral rhytids.
2. Prior to treatment, the patient's medical history should be obtained, and the patient should be fully apprised of the indications, contraindications, warnings, precautions, treatment responses, adverse reactions, and method of administration. Patients also should be advised that supplemental "touch-up" treatments may be required to achieve and maintain maximum correction.
3. The patient's treatment goals should be characterized with regard to proper proportion of upper and lower lip, vertical height, horizontal length, vermilion fullness, contouring of the vermilion border, Cupid's bow, and philtral columns, as well as perioral lip. rhytids and oral commissures. Pretreatment photographs are recommended.
4. Supplementary anesthesia may be used for additional pain management during and- after injection.
5. After ensuring that the patient has thoroughly washed the treatment area with soap and water, the area should be swabbed with alcohol or other antiseptic. Prior to injecting, depress the plunger rod until the product flows out of the needle.
6. After the first small amount of material has been injected into the patient, wait a full 3 seconds to allow the lidocaine to take effect before proceeding with the rest of the injection.
7. The injection technique may vary with regard to the angle and orientation of the bevel, the depth of injection, and the quantity administered. A tunneling technique, serial puncture. technique, fanning technique, or a combination has been used to achieve optimal results, Injecting the product too superficially may result in visible lumps and/or discoloration.
8. Inject Singderm® Silk by applying slow and even pressure on the plunger rod. It is important that the injection be stopped before the needle is pulled out of the skin to prevent material from leaking out or being placed too superfially in the skin.
9.If the needle is blocked, do not increase the pressure on the plunger rod. Instead, stop the
injection and replace the needle.
10. The typical volume injected into the lips and perioral area to achieve optimal correction was
approximately 2.6 ml, which may vary depending on the goals the patient wishes to achieve. Injection volumes into the lips and perioral area after repeat treatment tended to be lower, with the typical total injection volume to achieve optimal correction being approximately 1.6 mL.
11. Correct to 100% of the desired volume effect. Do not overcorrect. The degree and duration of the correction depend on the character of the defect treated, the tissue stress at the implant site, the depth of the implant in the tissue, and the injection technique. Markedly indurated defects may be difficult to correct.
12. If immediate blanching occurs, the injection should be stopped and the area massaged until it
returns to a normal color. Blanching may represent a vessel occlusion. If normal skin coloring does not return, do not continue with the injection. Treat in accordance with Society for Dermatologic surgery guidelines, which include hyaluronidase injection.
13. When injection is completed, the treated site should be gently massaged so that it conforms to the contour of the surrounding tissues. If overcorrection occurs, massage the area between your fingers or against underlying superficial bone/teeth to obtain optimal results.
14. With patients who have localized swelling, the degree of correction is sometimes difficult to judge at the time of treatment. In these cases, it is better to invite the patient back to the office for a touch-up treatment.
15. After the initial treatment, an additional touch-up treatment may be. necessary to achieve the desired level of correction. If further treatment is needed, the same procedure should be repeated until a satisfactory result is obtained. The need for an additional treatment may vary from patient to patient and is dependent upon a variety of factors such as treatment goals, lip fullness, perioral lines severity, skin elasticity, and dermal thickness at the treatment site.
16. Patients may have mild to moderate injection site responses after treatment in the lips and perioral area, which typically resolve within 14 days. Ice may be applied for a brief period following treatment to minimize swelling and reduce pain.
17. The health care professional should instruct the patient to promptly report to her/him any evidence of problems possibly associated with the use of Singderm® Silk.
C. Patient Instructions
It is recommended that the following information be shared with patients:
·Within the first 24 hours, patients should avoid strenuous exercise, extensive sun or heat exposure, and alcoholic beverages. Exposure to any of the above may cause temporary redness, swelling, and/or itching at the injection sites.
·To report an adverse reaction, contact the Singclean Product Support Department, http://www.singdean.net/
8. HOW SUPPLIED
Singderm® Silk injectable gel is supplied in individual treatment syringes with 30Gx1/2” needles for single-patient use and ready for injection (implantation). 1ml or 0.5ml. Singderm® Silk is
available. The volume in each syringe is as stated on the syringe label and on the carton. The contents of the syringe are sterile and non-pyrogenic. Do not resterilize. Do not use if package is opened or damaged.
9. STORAGE AND SHELF LIFE
Store at 2 °C to 30 °C. DO NOT FREEZE. Fragile.
Singderm® Silk shelf life is 24 months. Singderm® Silk injectable gel has a clear appearance. In the event that a syringe contains material that is not clear, do not use the syringe; notify Singclean
Product Support immediately at 86-571-6343-1868.
Latest version 8.12.04.343 A0 Issued Date: 2021-07-27