CE Singclean Multi-drug One Step Test Kit
(Colloidal Gold Method)
CE
Instructions for use for testing of any combination of the following drugs:
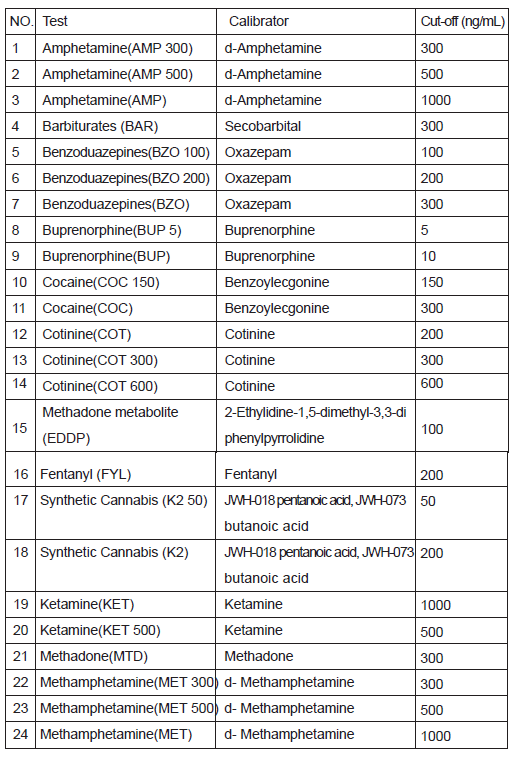
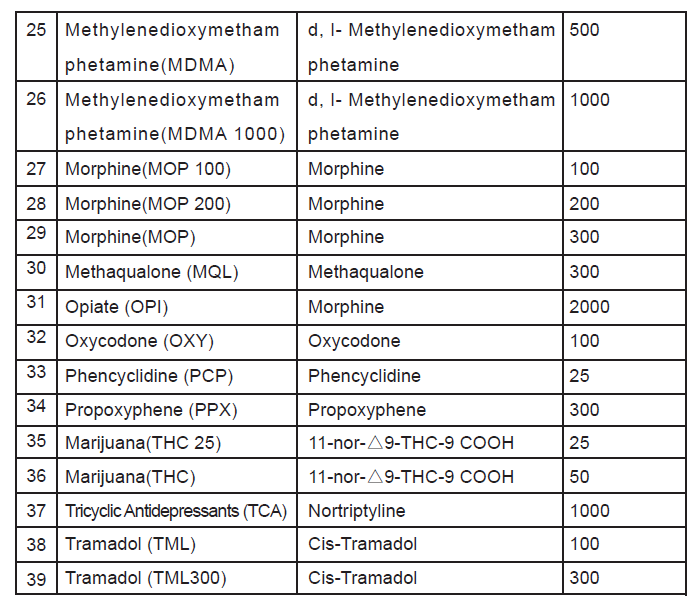
Amphetamine(AMP 300), Amphetamine(AMP 500), Amphetamine(AMP),Barbiturates(BAR), Benzoduazepines(BZO100), Benzoduazepines(BZO200),Benzoduazepines(BZO), Buprenorphine(BUP5), Buprenorphine(BUP), Cocaine(COC150), Cocaine(COC),Cotinine(COT), Cotinine(COT 300), Cotinine(COT 600), Methadone metabolite (EDDP),Fentanyl (FYL), Synthetic Cannabis (K2 50), SyntheticCannabis(K2),Ketamine(KET), Ketamine(KET 500), Methadone(MTD), Methampheamine(MET300), Methamphetamine(MET500), Methamphetamine(MET),Methylenedioxymethamphetamine(MDMA), Methylenedioxymethamphetamine(MDMA 1000), Morphine(MOP 100), Morphine(MOP 200), Morphine(MOP), Methaqualone (MQL), Opiate (OPI), Oxycodone (OXY), Phencyclidine (PCP),Propoxyphene (PPX),Marijuana(THC 25), Marijuana(THC), Tricyclic Antidepressants (TCA),Tramadol (TML), Tramadol (TML300).
Including specimen validity test (S.V.T.) for Oxidants/Pyridinium Chlorochromate(OX/PCC),specific gravity(SG), pH, Nitrite(NIT), Glutaraldehyde(GLUT) and Creatinine(GRE).
Intended use
The Multi-drug One Step Test Kit (Colloidal Gold Method) is a rapid chromatograpic
immunoassay for the qualitative detection for the drugs and drug metabolites in human urine. The detection limits cut-off concentrations of this test are as below list.
Special condition for use statement(s)
Configurations of multi-drug test kit come with any combination of the above listed drug analytes with or without specimen validity test (S.V.T.). This assay provides only a preliminary analytical test result. A more specific alternative chemical method must be used to obtain a confirmed analytical result. Gas chromatography/Mass spectrometry (GC/MS) is the preferred confirmatory method. Other chemical confirmation methods are available. Clinical consideration and professional judgment should be applied to any drug of abuse test result, particularly when preliminary positive results are used.
S.V.T Summary
Each S.V.T. strip contains chemically treated reagent pads. Three to five minutes following the activation of the reagent pads by the urine sample, the colours that appear on the pads can be compared with the printed colour chart card. The colour comparison provides a semi-quantitative screen for any combination of oxidants/pyridinium chlorochromate (PCC), specific gravity, pH, nitrite, glutaraldehyde and creatinine in human urine which can help assess the integrity of the urine sample.
Pack formats
Cassette: 25 tests/box
Panel: 25 tests/box
Cup: 20 tests/box
Principle
The multi-drug test kit is a competitive binding immunoassay in which drugs and drug metabolites in a urine sample compete with immobilized drug conjugate for limited labeled antibody binding sites. During testing, a urine specimen migrates through the test device by capillary action. If the drug or drug metabolite concentration in the specimen is below the cut-off level, the anti-drug antibodies in colloidal gold particles will bind to the drug antigens coated in the test line of the nitrocellulose membrane to form a T line which indicates a negative result. If the concentration of drug in the urine specimen is above the cut-off level, it will bind with antibodies conjugated with colloidal gold particles, so that no T line will be developed in the test region, which indicates a positive result. A control band with a different antigen/antibody reaction is added to the immune-chrom atographic membranestrip at the control region(C) to indicate that the test has performed properly. This control line should always appear regardless of the presence of drug or drug metabolite. If the control line does not appear the test device should be discarded.
Adulteration test Principle
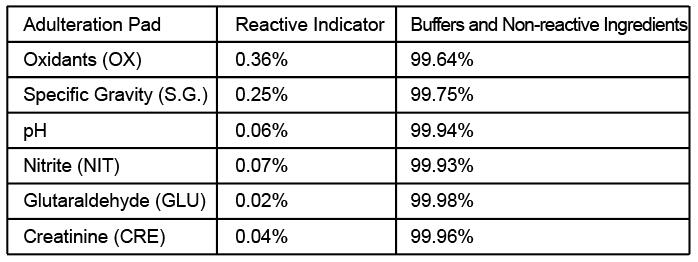
Adulteration is tampering of a urine specimen with the intention of altering the test results. The use of adulterants can cause false negative results in drug tests either interfering with test and/or destroying the drugs present in the urine. Dilution may also employed in an attempt to produce false negative results. One of the best way to test for adulteration or dilution is to determine certain urinary characteristics such as pH and specific gravity(SG) and Creatinine(GRE) and to detect the presence of Oxidants/Pyridinium Chlorochromate (OX/PCC), Nitrite(NIT), Glutaraldehyde(GLUT) in urine.
• Oxidants/PCC (Pyridinium chlorochromate) tests for the presence of oxidizing agents such as bleach and hydrogen peroxide. Pyridinium Chlorochromate is a commonly used
adulterant. Normal human urine should not contain oxidants or PCC.
• Specific gravity tests for sample dilution. The normal range is from 1.003 to 1.030.
Values outside this range may be the result of specimen dilution or adulteration.
• pH tests for the presence of acidic or alkaline adulterants in urine. Normal pH levels
should be in the range of 4.0 to 9.0. Values outside of this range may indicate the sample
has been altered.
• Nitrite tests for commonly used commercial adulterants such as Klear or Whizzies. They
work by oxidizing the major cannabinoid metabolite THC-COOH. Normal urine should contain no trace of nitrite. Positive results generally indicate the presence of an adulterant.
• Glutaraldehyde tests for the presence of an aldehyde. Adulterants such as Urin Aid
and Clear Choice contain glutaraldehyde which may cause false negative screening
results by disrupting the enzyme used in some immunoassay tests.2 Glutaraldehyde is
not normally found in urine; therefore, detection of glutaraldehyde in a urine specimen is generally an indicator of adulteration.
• Creatinine is a waste product of creatine, an amino acid contained in muscle tissue
and found in urine. A person may attempt to foil a test by drinking excessive amounts of
water or diuretics such as herbal teas to “flush” the system. Creatinine and specific gravity are two ways to check for dilution and flushing, which are the most common mechanisms used in an attempt to circumvent drug testing. Low creatinine and specificgravity levels may indicate dilute urine. The absence of creatinine (< 5 mg/dL) is indicativeof a specimen not consistent with human urine.
Precautions
• For in vitro diagnostic use only.
• For laboratory professional use.
• Do not use after the expiration date indicated on the package. Do not use the test if the foil pouch is damaged. Do not reuse tests.
• All specimens should be considered potentially hazardous and handle in the same
way as an infectious material.
• Avoid cross-contamination of specimens by using a new specimen collection
container and specimen pipette for each specimen obtained.
• Read the entire procedure carefully prior to performing any tests.
• The used testing materials should be discarded in accordance with local, state
and/or federal regulations.
• Any serious incident that has occurred in relation to the device should be reported
to the manufacturer and the competent authority of the Member State.
Storage and stability
• Store in the sealed pouch at 2℃ to 30℃.
• The test is stable through the expiration date printed on the sealed pouch.
• The test must remain in the sealed pouch until use.
• After opening the sealed pouch, use the test as soon as possible within 60 minutes.
• Do not freeze.
• Do not use beyond the expiration date.
Specimen collection and storage
• Fresh urine specimen must be collected in a clean and dry container. Urine collected
at any time of the day may be used. Urine specimens exhibiting visible particles should be centrifuged, filtered, or allowed to settle to obtain clear specimen for testing.
• Perform the testing immediately after the specimen collection. Do not leave the specimens at room temperature for prolonged periods. Specimens may be stored at 2-8°C for up to 48 hours. For long term storage, specimens should be kept below -20°C.
• Bring specimens to room temperature prior to testing. Frozen specimens should be
thawed and mixed well before testing. Avoid repeated freezing and thawing of specimens.
For best results, test specimens immediately following collection.
Materials
Materials Provided
• Sealed pouches each containing a test cassette/panel/cup, a desiccant
• S.V.T./Adulterant color chart( if applicable)
• Instructions for use
• Dropper(for cassette only)
Materials required but not supplied
• Specimen collection container
• Timer
Procedure
Equilibrate the tests, urine specimens, and/or controls to room temperature (15-25℃) prior to testing.
Remove the test from the sealed pouch and use it as soon as possible.
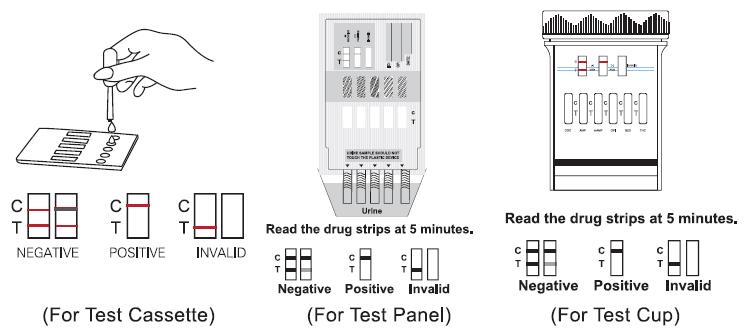
For Test Cassette
• Place the Test Cassette on a clean and level surface. Hold the dropper vertically and transfer 3 full drops of urine (approx.100L) to the specimen well (s). Avoid trapping air bubbles in the specimen well (S).
• Start the time and wait for the colored line to appear.
• Read the test results at 5 minutes. Do not read the results after 10minutes.
For Test Panel
• Remove the cap from the end of the test card. With arrows pointing toward the urine specimen, immerse the test panel vertically into the urine specimen for at least 10-15 seconds. Immerse the strip(s) at least the level of the wary lines, but not above the arrow(s) on the test card.
• Place the test panel on a flat dry surface, start the time and wait for the colored
line(s) to appear. Read the test results at 5 minutes. Do not read the results after 10minutes.
For Test Cup
•Collect specimen in the cup and secure the cap tightly.
•If the temperature strip is included with Drug Test Cup, please read urine temperature between 2-4 minutes after voiding to verify the temperature ranges between 32-38℃.
•Place the cup on a flat surface.
•Date and initial the security seal, and place the security seal on the cap.
•Peel off the label on the cup to view the results.
•Read the test results at 5 minutes. Do not read the results after 10minutes.
Note:
•If adulteration test is included, read the adulteration strip between 3 and 5 minutes.
See the color chart for interpretation. If the result indicates adulteration, do no interpret
the drug test result and either retest the urine or collect another specimen.
Interpretation of results
Negative
Two distinct colored lines appear. One line should be in the control line region (C) and
another line should be in the test line region (T).It indicates that the drug concentration in the urine specimen is below the designated cut-off level for the specific drug.
Positive
Only one colored band appears in the control region (C). No apparent colored band
appears in the test region (T). The absence of a line in the test region (T) indicates a
positive result. The positive result indicates that the drug concentration in the urine specimen is above the designated cut-off level for that specific drug.
Invalid
The result is invalid if no colored line appears in the control line region (C), even if a line appears in the test line region (T). You should repeat the test with a new test. Ifthe problem persists, discontinue using the lot immediately and contact your local distributor.
Adulteration interpretation
(Please refer to the color chart)Semi-quantitative results are obtained by visually comparing the reacted color blocks on the strip to the printed color indicator on the color chart. No instrumentation is required.
NOTE:
1.The intensity of the color in test region (T) may vary depending on the concentration
of aimed substances present in the specimen. If the color in test region (T) is very weak, indicating that the drug concentration in the urine specimen is close to the cut-off concentration, then the specimen needs to be detected repeatedly or confirmed by other more accurate method.
2.Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Quality control
• Internal procedural controls are included in the test. A colored band appearing in the control region (C) is considered an internal positive procedural control. It confirms sufficient specimen volume and correct procedural technique.
• External controls are not supplied with this kit. It is recommended that positive and negative controls be tested as a good laboratory practice to confirm the test procedure and to verify proper test performance.
Limitations
1.The multi-drug test kit provides only a qualitative, preliminary analytical result. A more specific chemical method must be used to obtain a confirmed result. Gas chromatography/Mass spectrometry (GC/MS) is the preferred confirmatory method.
2.It is possible that the technical or procedural errors, as well as other interfering substances in urine specimen may cause erroneous results.
3.The assay is designed for use with human urine only.
4.Adulterants, such as bleach and/or alum, in urine specimens may produce erroneous results regardless of the analytical method used. If adulteration is suspected, the test should be repeated with another urine specimen.
5.A positive result does not indicate level of intoxication, administration route or
concentration in urine.
6.A negative result may not necessarily indicate drug-free urine. Negative results can be obtained when drug is present but below the cut-off level of the test.
7.Test does not distinguish between drugs of abuse and certain medications.
8.Certain foods or food supplements may cause a false positive result.
Adulteration test limitations
1.The adulteration test with this product is mean to aid in the determination of abnormal specimens. While comprehensive, these tests are not mean to be an “all inclusive” representation of possible adulterants.
2.OX/PCC: Normal human urine specimen should not contain Oxidants and Pyridinium. Chlorochromate. The presence of high level of antioxidants in specimen, such as ascorbic acid, may result in false negative for the OX/PCC strip
3.SG: Elevated levels of protein in urine may cause abnormally high specific gravity values.
4.NIT: Nitrite is not a normal component of human urine. However, nitrite found inurine may indicate urinary tract infections or bacterial infections. Nitrite levels of >20mg/dL, may produce false positive glutaraldehyde result.
5.GLUT: Glutaraldehyde is not normally found in urine, however, certain metabolic abnormalities such as ketoacidosis (fasting, uncontrolled diabetes or high-protein diets) may interfere with test result.
6.GRE: Normal creatinine levels are between 20 and 350 mg/dL. Under rare
conditions, certain kidney diseases may show dilute urine.
Performance Characteristics
Accuracy
The accuracy of the Multi-drug One Step Test Kit was compared and checked against commercially available tests with a threshold value at the same cut-off levels. Urine samples taken from volunteers claiming to be non-users were examined under both tests. The results were >95% in agreement.
Analytical specificity
To test the specificity of the test, the test kit was used to test various drugs, drug metabolites and other components that are likely to be present in urine. All the components were added to drug-free normal human urine. These concentrations (ng/mL) below also represent the limits of detection for the specified drugs or metabolites.
Latest version 8.35.04.015 A0 lssued Date: 2021.03.15