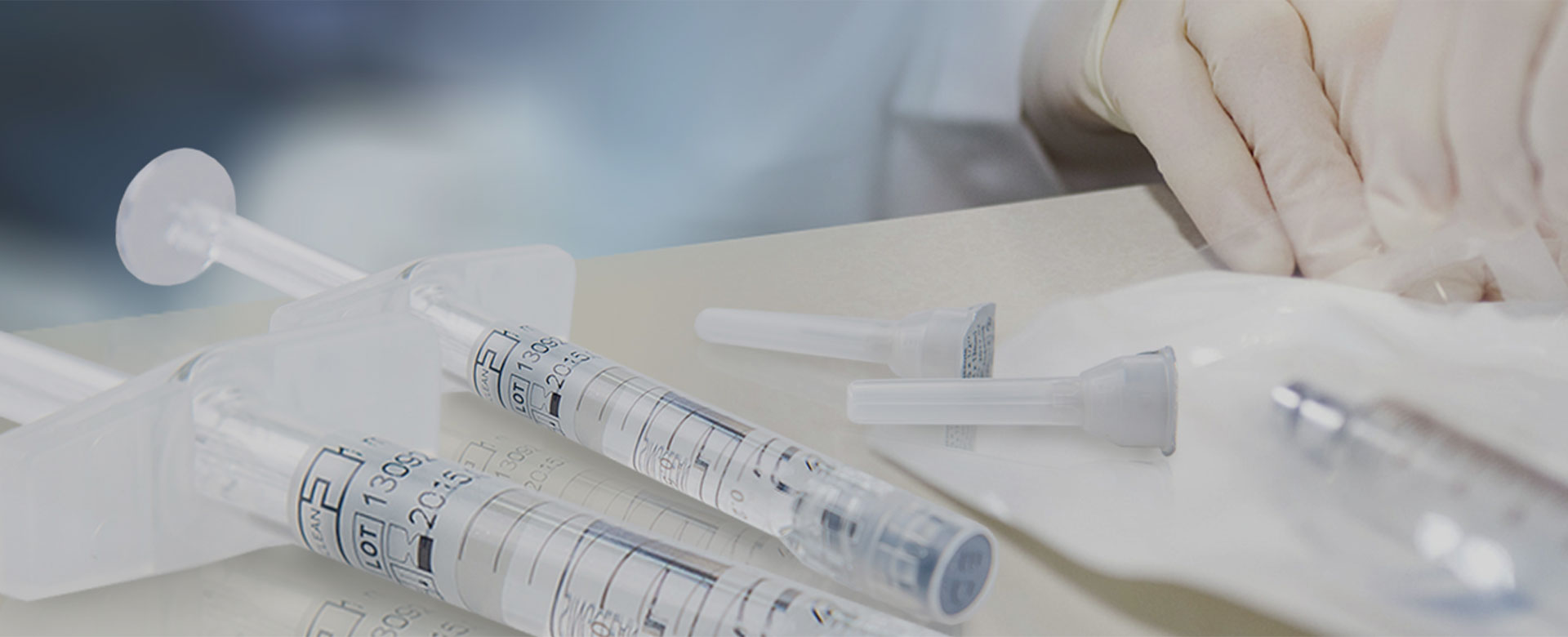
CE SingJoint® Medical Sodium Hyaluronate Gel (for Bone Joint)
CE 2460
[Primary ingredients and performance]
SingJoint® is a clear gel solution, obtained by dissolving sodium hyaluronate or hyaluronic acid(hereinafter as‘HA') in phosphate buffered saline,which is contained in a pre-filled syringe. SingJoint® is for single intra-articular injection. The product is a unique type of linear macromolecular mucopolysaccharide, composing repeating disaccharide units of glucuronic acid and N-acetyl glucosamine. Its chemical formula is (C14H21NO11)n.
SingJoint® , is non-animal original, with the advantages of high viscoelasticity, lubricity, physical alterability and good biocompatibility. It is a medical polymer extracted and refined by high-tech bio-engineering from streptococcal fermentation metabolites. It is sterile, and free from pyrogenicity, allergy, genetic toxicity, or skin irritation. Due to fermentation production of HA, the product has no risk of being a virus carriers and dissemination medium when extracting animal tissues or organs. This product can be used as an orthopedics agent to improve the joint function. When used for injections in joint cavities, due to its macromolecular characteristic and good viscoelasticity, it stimulates synovium to produce high molecular sodium hyaluronate, which is beneficial for releasing joint pain, increasing mobility, decreasing synovitis and delaying progression of disease.
[Indications]
SingJoint® is indicated for the treatment of symptoms of osteoarthritis of knee. By replacing and supplementing the pathological synovial fluid in the osteoarthritic joints, it reduces pain and improves joint function.
[Uses]
A. To Attach Needle to Syringe
1.Remove the tip cap. Hold syringe and pill tip cap off the syringe.
2.Insert needle. Hold the syringe body and firmly insert the hub of the needle (select a suitable puncture needle) into the luer-lock at the end of the syringe.
3.Tighten the needle. Tighten the needle by turning it firmly in a clockwise direction until it is seated in the proper position.
B. Physician instructions
1.As SingJoint® is administered by intra-articular injection, the injection of SingJoint® should be performed only by trained physicians.
2.Aseptic injection procedure shall be followed.
3.If joint effusion presents, it should be aspirated before performing the injection. The dosage regimen is injecting the product into the affected synovial joint cavity by one injection.
4.Discard the syringe and needle after the single use.
The dosage regimen is injecting into the affected synovial joint space once a week with a total of three to four injections.
[Adverse Reactions]
Mild transient local pain, swelling, arthralgia and joint stiffness at the injection site are reported after intra-articular injections. These symptoms are generally mild and transient. They usually resolve within 2 to 7 days without medical intervention. If symptoms are persistent, please consult with your treatment doctor.
[Precautions]
1.Strict aseptic technique must be followed for preventing patients from Cross infection;
2.The pre-filled syringe is intended for single use. The contents of the syringe must be used immediately after opening the packaging. Discard any rest part of SingJoint®. Secondary use is possible to lead to some infectious diseases;
3.Do not use if the blister package is in damaged condition, or if any cracks or breakage appears on the pre-filled syringe;
4.Do not inject SingJoint® into vessel;
5.Do not inject SingJoint® out of joint cavity or into the synovial tissue or capsule;
6.If joint infection presents, infection should be eliminated before injecting SingJoint®;
7.Do not use disinfectants containing quaternary ammonium salts simultaneously for skin preparation, since sodium hyaluronate may precipitate when meet this ingredient.
[Contraindications]
1.SingJoint® is contraindicated for patients with known hypersensitivity (allergy) to SingJoint® or any other sodium hyaluronate preparations;
2. SingJoint® is contraindicated for patients with skin diseases or infection in the area of injection site;
3. SingJoint® is contraindicated for patients with acute and chronic pyogenic arthritis, synovium and joint tuberculosis, haemorrhagic diseases and haemophilia.
[Notes]
The container for medical sodium hyaluronate is composed by glass. Please use carefully.
Do not use after the expiry date printed on the package. The expiry date refers to the product stored in its original packaging at the temperature between 2°C -20°C.
Keep out of reach of children.
The safety and effectiveness of SingJoint® has not been tested on pediatric patients, pregnant or lactating woman.
[Storage]
Store between 2°C and 20°C, do not freeze.
Relative humidity shall be no more than 80%.
Store in a clean, well ventilated condition, and avoid corrosive gas.
Protect from light.
[Shelf life]
2 years
[Specifications]
10 mg/mL: 1.0 mL, 2.0 mL.2.5 mL; (10 mg/1mL,20 mg/2mL, 25 mg/2.5mL)
12 mg/mL: 1.0 mL, 2.0 mL. 2.5 mL; (1 2 mg/1mL, 24 mg/2mL, 30 mg/2.5mL)
Latest version 8.03.04.0322 G6 lssued date: 2025-06-18