CE Singclean® (Medical sodium hyaluronate gel)
Viscoelastic Solution for Ophthalmic Surgery
CE 2460
Description:
Singclean® (Medical sodium hyaluronate gel) is clear liquid gel,which is compounded sodium hyaluronate and physiological balancing salt. Hyaluronic Acid (herein after 'HA') is a unique type of linear macromolecular mucopolysaccharide, composed of repeating disaccharide units of glucuronic acid and N-acetylglucosamine, in chemical formula of (C14H21NO11)n. Singclean® is a high molecular weight (between 1,000,000~2,600,000) fraction of sodium hyaluronate (1.5% total weight) dissolved in a sodium phosphate buffer. The osmotic pressure of Singclean® is nominally 270 mOsmol/kg ~ 350mOsmol/kg.
Indications:
Singclean® is intended to aid anterior or posterior segment ophthalmic surgery, including:
·Cataract extraction with/without implantation of intraocular lens;
·Corneal transplant surgery;
·Glaucoma filtering surgery;
·Secondary lens implantation.
Contraindications:
Patients with allergy background should not use this product.
Precautions:
Overfilling the eye anterior or posterior segment with Singclean® may lead to increased intraocular pressure, glaucoma, or other ocular damage.
Postoperative intraocular pressure may also be elevated as result of pre-existing glaucoma, compromised outflow and by operative procedure and sequel a thereto, including enzymatic zonulysis, absence of an indectomy, trauma to filtration structures, and by blood and lenticular remnants in the anterior chamber.
It is difficult to predict the exact role of these factors in any individual case, therefore the following precautions are recommended:
·Never overfill the eye chambers with Singclean® (except in glaucoma surgery).
· In posterior segment procedures in the aphakic diabetic patients, special care should be exercised to avoid using large amounts of Singclean®.
·Remove all remain Singclean® by irrigation and /or aspiration at the close of surgery (except in glaucoma surgery).
·Carefully monitor the intraocular pressure especially during the immediate postoperative period. If significant rises are observed, treat with appropriate therapy.
·Singclean® is obtained from microbial fermentation by a highly purified proprietary process. The physician should be aware of potential allergic risks that can occur with the injection of any biological material.
·Needle is for single use only.
·Avoid trapping air bubble.
·On rare occasion, viscoelastic Singclean® containing sodium hyaluronate has been observed to become slightly opaque or to form a slight precipitated upon instillation into the eye. Should this be observed, the cloudy or precipitated material should be removed by irrigation and/or aspiration.
·Use only if solution is clear.
·The graduation scale on the syringe label is only indicative.
Adverse reactions:
Singclean® is extremely well tolerated after injection into human eyes. A transient rise of intraocular pressure postoperatively has been reported in some cases. In posterior segment surgery intraocular pressure rise have been reported in some patients, especially in aphakic diabetics, after injection of large amounts of Singclean®.
Rarely, postoperative inflammatory reactions (iritis, hypopyon) as well as incidents of corneal edema and corneal decompensation have been reported. Their relationship to Singclean® has not been established.
Application:
·Cataract surgery and IOL implantation
The required amount of Singclean® is slowly infused through a needle or cannula into the anterior chamber. The protective effect of Singclean® as an aid is optimized when the injection is performed prior to cataract extraction and insertion of the IOL and is effective for both intra- and extra-capsular cataract procedures. Singclean® may be applied to IOL prior to insertion. Additional Singclean® can be injected as required to facilitate surgical procedures.
·Corneal transplant surgery
The corneal button is removed and the anterior chamber filled with Singclean® is at level with the surface of the corneal. The donor graft is then placed on top of the Singclean® and sutured into place. Additional Singclean® can be used as required to aid in surgical procedures.
·Glaucoma filtration surgery
Singclean® is injected through a corneal paracentesis to restore and maintain anterior chamber volume during the performance of the trabeculectomy. Additional Singclean® can be used as required to aid in the surgical procedures.
Direction for Use:
First expulse the air out of the ophthalmic Luer-lock needle , then inject Singclean® appropriately into eye. The injected amount must be adapted to the type of surgical procedures. The administration of Singclean® should be performed only by trained specialists.
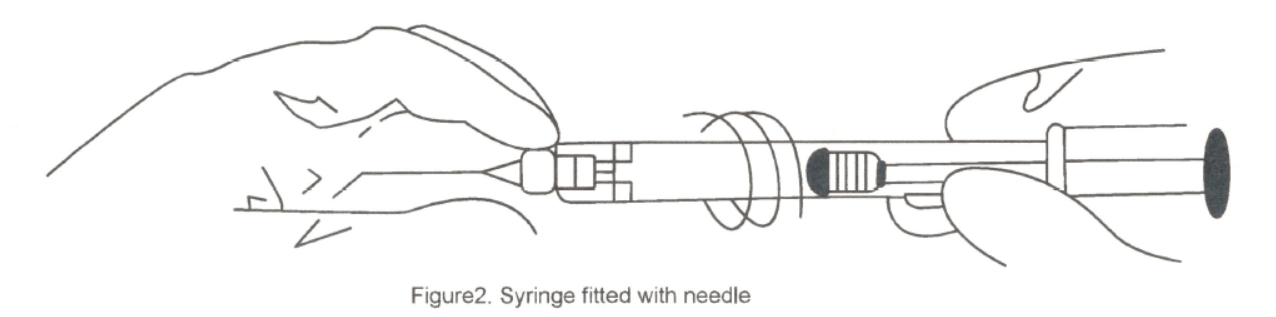
The needle should fit to the syringe luer tip (shown in figure 2); however, over tightening may cause the hub to weaken and possibly detach from the syringe. Extrusion of a test droplet is recommended prior to entering eye, and excessive force on the plunger should be avoided.
Interactions:
There is an incompatibility between sodium hyaluronate and solutions containing quaternary ammoniums, like benzalkonium chloride. In that case Singclean® shall not be in contact with medico chirurgical materials rinsed with such a solution or with ophthalmic topics containing a quaternary ammonium as a preserving agent.
Note:
·The product is sterilized and free from pyrogenicity, and must be operated in a sterile environment;
·It is for single use only. Do not use if sterile packaging has been damaged;
·The used and expired products should be disposed in compliance with pertinent government regulations regarding medical devices;
·The syringe is composed by glass. Be careful in using;
·Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because hyaluronan can precipitate in their presence;
·Must not be used for intravenous injection;
·The user must be trained to obtain relevant qualifications before using;
·ln case of any serious incident related to the device (especially adverse events), it shall be reported to the manufacturer or the EU authorised representative or the competent authority;
·Pay attention to the physical injury caused by needles.
Model:
15mg/mL: 0.5mL, 0.75mL, 1.0mL, 2.0mL
Storage:
Store between 2℃ and 20℃, do not freeze. Relative humidity no more than 80%, well ventilated and clean, no corrosive gas, protect from light. Do not injection after expiry date.
Latest version 8.02.04.0331 F4 Issued Date: 2024-06-17